Fig. 8.
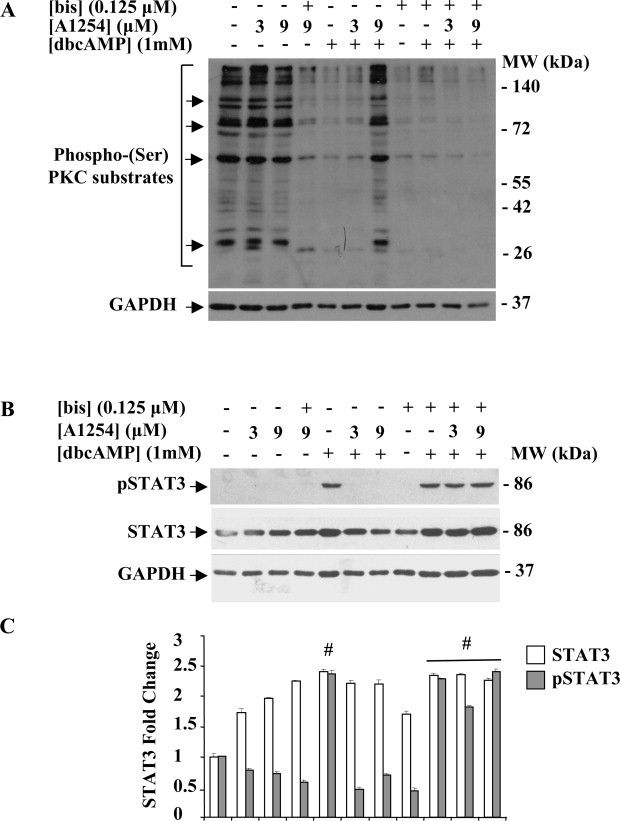
Effects of A1254 treatment on PKC activity (A) and activation status of STAT3 (B) in bis co-treated dbcAMP C6 cells. C6 cells were treated or not with A1254 (3 or 9 μM) in presence or absence of the PKC inhibitor, bis (0.125 μM) during dbcAMP (1 mM) stimulation in serum-deprived medium containing 0.1% (v/v) DMSO, used as vehicle for A1254 and bis. After 24 h incubation, control and treated cells were harvested and equal amounts of protein cell lysates (20 μg) were separated by 12% SDS–PAGE and subjected to immunostaining, as described in Section 2.5. (A) Western blotting using an anti-phospho-(Ser) PKC substrates showing PKC substrates phosphorylation. Arrows indicated the more abundant phosphorylated protein bands (Mr of 200, 78, 65 and 28 kDa). (B) Western blotting showing phospho-Serine727 form of STAT3 and STAT3 total protein expression levels. GAPDH was used as loading control for cell lysates. Signals were revealed by immunostaining and ECL, as described in Section 2.5. (C) Densitometric analysis of phospho-STAT3 and total protein expression levels; fold changes were calculated by first normalizing to GAPDH levels in individual samples and then relative to untreated control (cells cultured in serum-free DMEM with 0.1% (v/v) DMSO, vehicle) set as 1. Each bar represents the mean ± SEM (n = 3). Columns with (#) were statistically different from untreated control or dbcAMP-differentiated cells (#p < 0.001).
