Fig. 2.
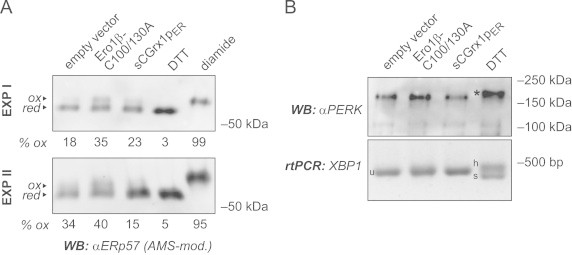
sCGrx1pER neither causes ER hyperoxidation nor ER stress. (A) HeLa cells were transfected with the indicated constructs for 24 h or, to obtain fully reduced or oxidized control samples, treated with DTT (10 mM for 5 min) or diamide (5 mM for 5 min), respectively. Free cysteines were alkylated in situ with NEM. After cell lysis, proteins were reduced with TCEP and re-alkylated with AMS, and the modified lysates analyzed by SDS-PAGE and anti-ERp57 western blot (WB). The AMS-modified, oxidized fraction of ERp57 (ox) runs slower than the reduced fraction (red). Oxidized fractions, as determined by densitometry, are indicated in percent (% ox). Results of two independent experiments (EXP I and EXP II) are shown. (B) HeLa cells were transfected as in panel (A) or treated with DTT (2 mM for 1 h). The phosphorylation/activation of PERK was analyzed by anti-PERK western blot based on the decreased mobility of the phosphorylated protein (asterisk). In equivalent cell samples, total RNA was isolated and subjected to rtPCR analysis using primers specific for XBP1. Splicing of XBP1 mRNA is evident by the appearance of the spliced (s) and the hybrid (h) forms, as opposed to the unspliced (u) form.
