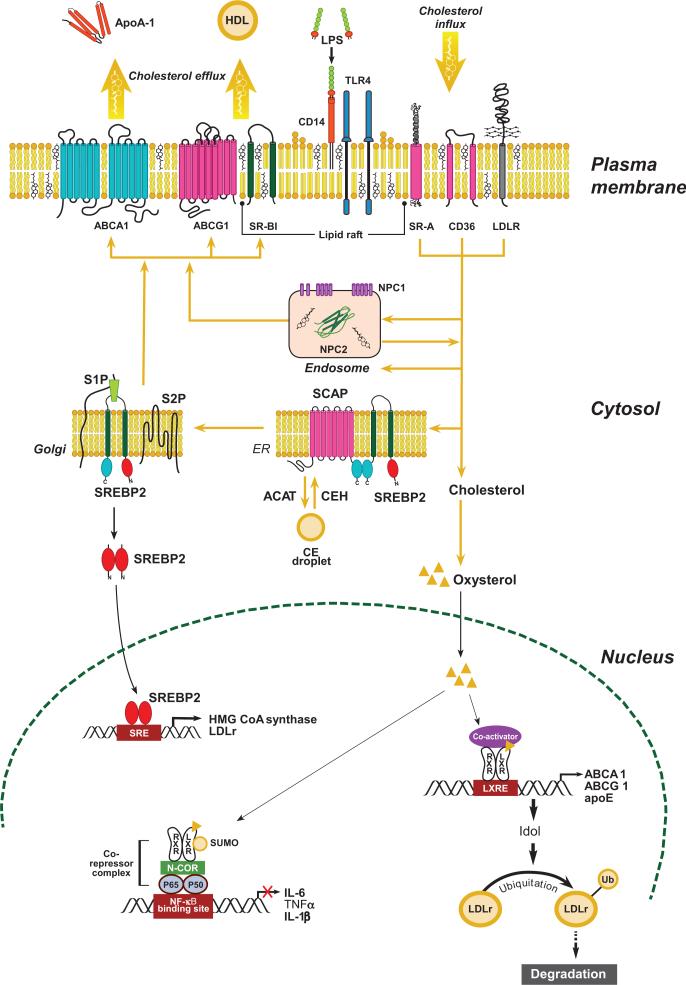
Figure 2.
Regulation of intracellular cholesterol homeostasis. Cellular cholesterol content is normally tightly regulated by balancing uptake and endogenous synthesis with efflux and storage. Cholesterol in lipoproteins is internalized via scavenger receptors (CD36, SR-A) or low-density lipoprotein receptors (LDLr) and delivered to endosomes where cholesteryl ester (CE) is hydrolyzed to free cholesterol (FC). Niemann-Pick C1 (NPC1) together with NPC2 regulates FC movement out of the endosomes for delivery to the endoplasmic reticulum (ER) or the plasma membrane. Excess FC in the ER can be esterified to CE by acyl CoA:cholesterol acyltransferase (ACAT), forming a cytosolic lipid droplet. CE can be mobilized from the lipid droplet by cholesterol ester hydrolase (CEH) for transport to the plasma membrane. When cellular cholesterol level is low, sterol regulatory element-binding protein 2 (SREBP2) is escorted by SREBP cleavage-activating protein (SCAP) from the ER to the Golgi, where it undergoes cleavage by site 1 and site 2 proteases (S1P and S2P). The proteolytic processing of SREBP2 releases the N-terminus, which is a basic helix-loop-helix leucine zipper transcription factor that travels to the nucleus to activate transcription of genes involved in cholesterol biosynthesis and cholesterol uptake (i.e., LDLr). A minor amount of FC can be oxidized into oxysterol, an activating ligand for liver X receptors (LXRs), resulting in increased transcription of cholesterol efflux genes (i.e., ABCA1, ABCG1, and apoE) and Idol (inducible degrader of LDLr), which mediates degradation of the LDLr. FC transported to the plasma membrane can be effluxed to lipid-free apolipoproteins (i.e., apoA-I and apoE) by ABCA1 or to high-density lipoprotein (HDL) particles by passive diffusion or transporters (ABCG1 or SR-BI). Modified from (31).