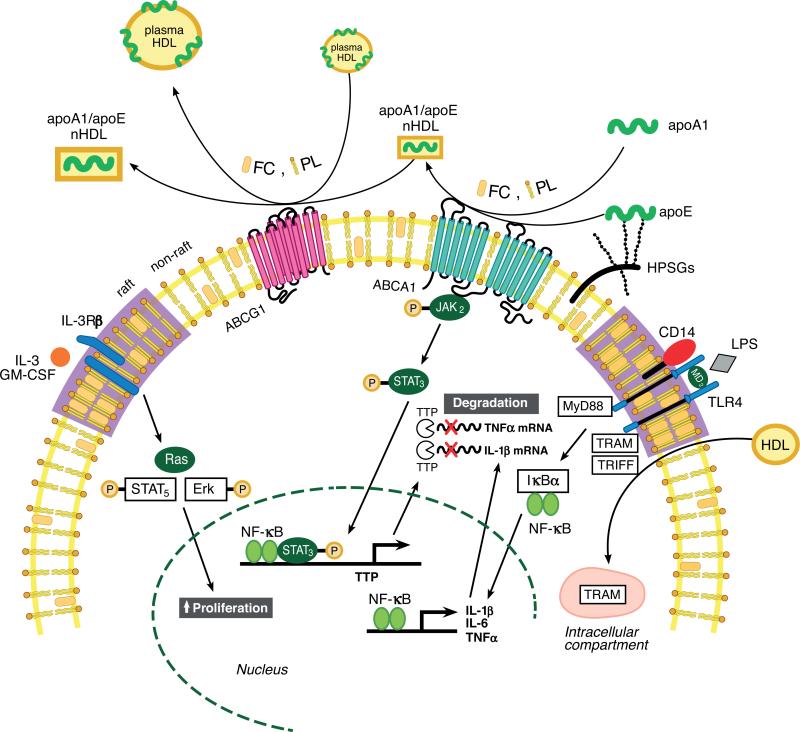
Figure 3.
Summary of roles of high-density lipoprotein (HDL), apolipoproteins, and ABC transporters in regulating inflammatory response and hematopoietic stem cell proliferation. Lipid-poor apoA-I or cell surface proteoglycan-bound apolipoprotein (apo)E stimulates free cholesterol (FC) and phospholipid (PL) efflux from macrophages or hematopoietic stem cells via ATP-binding cassette transporter (ABC) A1 to form nascent HDL (nHDL) particles. nHDL or plasma HDL can efflux additional FC and PL from the cells via ABCG1, forming FC-enriched nHDL and plasma HDL, thereby reducing cellular cholesterol levels. The reduction in membrane FC content mediated by ABCA1 and ABCG1 efflux results in reduced membrane lipid raft content. This, in turn, reduces inflammatory and proliferative pathways by reducing Toll-like receptor (TLR) 4 receptor and the common beta subunit of the interleukin (IL)-3/granulocyte macrophage colony-stimulating factor (GM-CSF) receptor (IL-3Rβ) in lipid rafts on the macrophage and hematopoietic stem cell surface, respectively. HDL also inhibits lipopolysaccharide (LPS)-stimulated type I interferon response in macrophages by inducing sequestration of TLR4 adapter TRIF-related adapter molecule (TRAM) in an intracellular compartment in a sterol-independent manner. Interaction of apoA-I with ABCA1 activates Janus kinase 2/signal transducer and activator of transcription 3 ( JAK2/STAT3) and increases transcription of tristetraprolin (TTP), resulting in destabilization of microRNAs of proinflammatory genes. Modified from (61, 64).