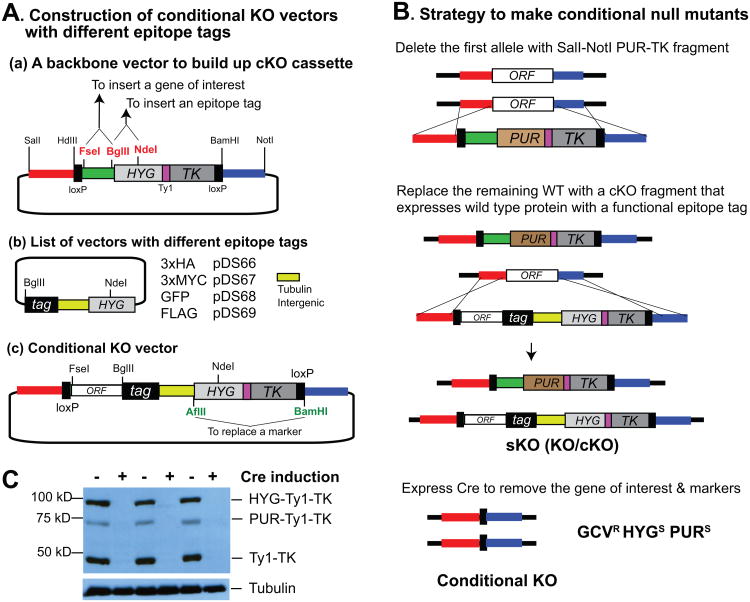
Fig. 2. Construction of a conditional null mutant.
(A) Construction of a conditional KO vector. The coding region of a gene can be inserted into the KO vector between FseI and BglII sites without or with (if epitope tagging is not desired) a stop codon. BglII and NdeI sites can be used to add different epitope tags. Between the tag and HYG is a TUB intergenic region, to provide UTRs for the gene of interest, and HYG. The final cKO cassette will have a loxP–ORF–epitope–3′ UTR–5′ UTR–HYG-TK–loxP so the gene of interest and the marker can be removed by Cre expression. Cloning at AflII and BamHI sites can be used to replace markers. (B) Strategy to make a conditional null mutant. One allele is removed with the PUR-TK KO vector. The remaining wild-type allele can be replaced with a cKO cassette that expresses wild-type allele with various epitope tags. Targeting can be confirmed by PCR or Southern blot. Unlike with the KO strategy, recombination can also occur between ORFs resulting in loss of the upstream loxP site, in which case Cre-loxP removal will not work. Therefore, it is essential to verify that both loxP sites are present after targeting. Cre expression will remove the region between the loxP sites. (C) Loss of markers by Cre expression. Gene knockout can be confirmed by western blot using antibodies against epitopes fused to the wild-type allele and by monitoring the loss of markers, which contain the Ty1 epitope sequence. The western blot shows that marker proteins disappeared after Cre induction. Tubulin was used as a loading control.