Abstract
In biology, homeostasis refers to how cells maintain appropriate levels of activity. This concept underlies a balancing act in the nervous system. Synapses require flexibility (i.e. plasticity) to adjust to environmental challenges. Yet there must also exist regulatory mechanisms that constrain activity within appropriate physiological ranges. An abundance of evidence suggests that homeostatic regulation is critical in this regard.
In recent years, important progress has been made towards identifying molecules and signaling processes required for homeostatic forms of neuroplasticity. The Drosophila melanogaster third instar larval neuromuscular junction (NMJ) has been an important experimental system in this effort. Drosophila neuroscientists combine genetics, pharmacology, electrophysiology, imaging, and a variety of molecular techniques to understand how homeostatic signaling mechanisms take shape at the synapse. At the NMJ, homeostatic signaling mechanisms couple retrograde (muscle-to-nerve) signaling with changes in presynaptic calcium influx, changes in the dynamics of the readily releasable vesicle pool, and ultimately, changes in presynaptic neurotransmitter release.
Roles in these processes have been demonstrated for several molecules and signaling systems discussed here. This review focuses primarily on electrophysiological studies or data. In particular, attention is devoted to understanding what happens when NMJ function is challenged (usually through glutamate receptor inhibition) and the resulting homeostatic responses. A significant area of study not covered in this review, for the sake of simplicity, is the homeostatic control of synapse growth, which naturally, could also impinge upon synapse function in myriad ways.
1. The Drosophila NMJ exhibits robust homeostatic control of synaptic function
Biological systems require physiologically tolerable ranges of activity. By definition, homeostatic mechanisms must respond to challenges that could push activity outside of acceptable ranges. The nervous system is no exception. At the level of synapses and circuits, examples of robust homeostatic plasticity have been characterized by many labs employing a panoply of invertebrate and vertebrate experimental systems (Davis, 2006; Marder, 2012; Marder and Goaillard, 2006; Perez-Otano and Ehlers, 2005; Pozo and Goda, 2010; Turrigiano, 2012; Turrigiano, 2008). A major task is to delineate the signaling processes that drive synaptic homeostasis – and to identify which ones are universally conserved. Based on current progress, studies at the Drosophila melanogaster neuromuscular junction (NMJ) promise to be valuable.
The Drosophila NMJ is a glutamatergic synapse that is readily accessible for electrophysiological analysis (Jan and Jan, 1976a, b) (Figure 1A). The NMJ exhibits a strong homeostatic response to changes in excitability (Figure 1B). The main type of perturbation or “homeostatic challenge” employed at this synapse is impairment of postsynaptic glutamate receptor function. Pioneering studies in the 1990s demonstrated that deletion of a Drosophila glutamate receptor subunit gene (GluRIIA) (DiAntonio et al., 1999; Petersen et al., 1997) and muscle-specific expression of constitutively active Protein Kinase A (PKA) (Davis et al., 1998) greatly diminish muscle response to single vesicles of glutamate (quantal size). The NMJ responds to this decrease in quantal size with a robust, homeostatic increase in presynaptic neurotransmitter release (quantal content). This increase in quantal content restores evoked muscle responses to normal levels (Davis et al., 1998; DiAntonio et al., 1999; Petersen et al., 1997) (Figure 1B). PKA-mediated diminishment of quantal size requires GluRIIA-containing receptors, suggesting that both genetic perturbations act through the same mechanism (Davis et al., 1998).
Figure 1. The Drosophila NMJ exhibits robust homeostatic control of synaptic function.
A) A series of images displaying the Drosophila larval NMJ preparation. A larva is pinned at its anterior and posterior, a shallow dorsal incision is made (dotted line), and a hexagonal filet exposes muscles and nerves for electrophysiological analysis or staining. Bouin’s fixative is added to this preparation to better visualize the muscles. The final image of the fixed preparation shows a close-up view of the central nervous system (CNS) and adjacent muscles. B) A basic cartoon model of how homeostatic upregulation of neurotransmitter release proceeds at the Drosophila NMJ. The muscle is gray and the neuron is blue. Only a few key components are shown. Pharmacological or genetic inhibition of muscle glutamate receptors greatly diminishes quantal size. This is evident from decreases in spontaneous miniature excitatory postsynaptic potentials (small traces). However, relatively normal evoked potentials (large traces) are achieved because of homeostatic, muscle-to-nerve retrograde signaling processes (RS - green arrows) that increase quantal content. At least two mechanisms facilitate this increase in quantal content: 1) increased presynaptic calcium influx (presumably through CaV2-type calcium channels); and 2) increased size of the readily releasable pool of synaptic vesicles.
The GluRIIA and GluRIIB glutamate receptor subunit genes are redundant for fruit fly viability, but the corresponding gene products behave differently electrophysiologically (DiAntonio et al., 1999). Decrease in quantal size – and therefore homeostatic pressure to increase presynaptic glutamate release – appears to occur specifically when GluRIIA-containing receptors are eliminated or reduced (DiAntonio et al., 1999; Petersen et al., 1997). Of note, when levels of the essential GluRIII (also known as GluRIIC) glutamate receptor subunit are lowered, one also observes greatly diminished quantal size, accompanied by enhanced quantal content (Marrus et al., 2004).
Similar results have corroborated these original findings. Postsynaptic factors that ensure proper levels of glutamate receptor clustering reveal the homeostatic properties of the NMJ. For example, loss-of-function mutations in Drosophila p21 activated kinase (Pak) (Rho-type GTPase effector gene) (Albin and Davis, 2004), mutations in dorsal and cactus (NFκB and IκB genes) (Heckscher et al., 2007), or loss of the translational repressor gene nanos (Menon et al., 2009) all diminish glutamate receptor clusters at the NMJ. In each case, the NMJs of mutants show reduced quantal size, coupled with homeostatically increased quantal content (Albin and Davis, 2004; Heckscher et al., 2007; Menon et al., 2009).
However, there are exceptions, and these exceptions may lead to new information about how homeostatic signals are propagated. For instance, the Drosophila Neto protein (Neuropilin and Tolloid-like) is required to cluster glutamate receptors at the NMJ (Kim et al., 2012). Hypomorphic neto mutant larvae can survive, but by electrophysiology, their NMJs display dramatic decreases in quantal size (Kim et al., 2012). However, there is no associated homeostatic increase in quantal content (Kim et al., 2012). One possibility that could explain these results is that Neto-mediated signaling processes are required for both proper glutamate receptor clustering and for propagation of retrograde homeostatic signals. Follow-up experiments are needed to test this idea.
2. Rapid synaptic homeostasis by pharmacology
Genetic impairments of glutamate receptor function last throughout development. For a third instar Drosophila larva raised at 25° C, this developmental process takes less than a week. But how quickly does the NMJ respond to a challenge to its glutamate receptor function? Is it a process that must take place over a period of days? Or is it more rapid? To measure the speed of homeostatic responses, it has been helpful to exploit pharmacological reagents that challenge synapse function on a short timescale.
It is known that invertebrate glutamate receptor function can be blocked with drugs such as Argiotoxin-636, which is derived from spider toxin (Broadie and Bate, 1993; DiAntonio et al., 1999; Jackson and Usherwood, 1988; Jarecki and Keshishian, 1995; Zhong and Pena, 1995). Another effective glutamate receptor blocker is Philanthotoxin-433 (PhTx), a non-competitive, open channel blocker derived from wasp venom (Eldefrawi et al., 1988; Karst and Piek, 1991). Injections of sub-blocking concentrations of PhTx into third instar Drosophila larvae paralyzes them for about 30 minutes, but then they recover the ability to move (Frank et al., 2006). Electrophysiological analyses of the NMJs of injected animals reveal decreased quantal size with homeostatically increased quantal content (Frank et al., 2006), reminiscent of GluRIIA mutant NMJs (DiAntonio et al., 1999; Petersen et al., 1997).
The fact that PhTx-injected larvae regain movement and show robust evoked electrophysiological responses on a time scale of minutes (as opposed to hours or days) points to a rapid system of compensation at the NMJ. Acute toxin exposure bears this out: Direct applications of PhTx-433, PhTx-343, and the spider neurotoxin NSTX-3 to dissected NMJ preparations reveal that significant homeostatic compensation can occur within ten minutes; and pharmacogenetic data suggest that PhTx works by specifically impairing GluRIIA-containing receptors (Frank et al., 2006). Surprisingly, this rapid form of homeostatic compensation can occur in the absence of either protein translation or evoked neurotransmission (Frank et al., 2006).
If evoked neurotransmission is not required, then what is triggering the homeostatic signaling process to begin? One possibility is that the approximately 2000 spontaneous miniature release events that occur in ten minutes of PhTx exposure communicate sufficient information (Frank et al., 2006). The idea that spontaneous miniature events could shape the time course and robustness of homeostatic signaling mirrors data from rodent hippocampal neurons and slices in which NMDA receptor blockade induces a rapid form of synaptic scaling (Sutton et al., 2006). However, one notable difference is that rapid homeostatic scaling in the rodent system proceeds via a protein-synthesis-dependent mechanism (Sutton et al., 2006).
Do the acute (PhTx) and chronic (GluRIIA mutant) challenges to neuromuscular function induce homeostatic increases in quantal content through identical signaling mechanisms? This question is still being investigated, but it seems unlikely. Several factors appear to control the sustained expression of synaptic homeostasis throughout life, but not the rapid induction of it. The existence of such molecules likely means that some signaling events need to occur rapidly in response to an acute homeostatic challenge – and then over time, additional factors are required for the NMJ to consolidate that response.
3. Alternative modes of triggering homeostatic signaling mechanisms
Is there only one way to induce a homeostatic response at the Drosophila NMJ – postsynaptic inhibition of glutamate receptors that results in increased presynaptic release? No. There are several perturbations that do not target the glutamate receptors directly but nevertheless induce homeostatic forms of compensation.
A manipulation independent of glutamate receptor function is general impairment of muscle excitability. Postsynaptic expression of the Kir2.1 potassium channel impairs muscle depolarization (Paradis et al., 2001). Kir2.1-expressing muscles have severely reduced input resistance and hyperpolarized resting potentials (Paradis et al., 2001). Increased quantal content compensates for reduced muscle excitability, reminiscent of GluRIIA mutant NMJs; however, in contrast to GluRIIA mutant NMJs, voltage clamp recordings reveal that there does not appear to be any measurable deficit in glutamate receptor function in the Kir2.1 muscles (Paradis et al., 2001).
Another perturbation is to alter the amount of innervation that individual muscles receive. This has been achieved by misexpressing the cell adhesion molecule Fasciclin II (Davis et al., 1997; Grenningloh et al., 1991; Lin and Goodman, 1994). In an elegant set of experiments, electrophysiological recordings were performed on two neighboring muscles that were either hyper-innervated or hypo-innervated due to misexpression of Fasciclin II (Davis and Goodman, 1998). Single bouton recordings were also performed. Despite having dramatically increased numbers of synaptic boutons, hyper-innervated muscles exhibit normal electrophysiological properties because the probability of release at single boutons is homeostatically downregulated (Davis and Goodman, 1998). By contrast, recordings from hypo-innervated muscles reveal increases in quantal size, accompanied by reduced quantal content (Davis and Goodman, 1998). In sum, the Drosophila NMJ is capable of homeostatically responding to multiple perturbations of function – not simply the loss of glutamate receptor function – and possibly through a diverse array of mechanisms.
4. Is it possible to downregulate neurotransmitter release by homeostatic mechanisms
Decreased glutamate receptor function (GluRIIA mutation or PhTx) and decreased muscle excitability (Kir2.1 overexpression) are sufficient to induce homeostatic increases in presynaptic glutamate release. Is the opposite true? Do increases in quantal size directly correlate with decreases in quantal content at the Drosophila NMJ? This appears to be the case with hypo-innervated muscles (Davis and Goodman, 1998), but more generally, do insect neuromuscular synapses have built-in safeguards against excessive neurotransmission? The data are mixed.
One example of homeostatic downregulation of synaptic function involves overexpression of the Drosophila vesicular glutamate transporter (DVGLUT) in motor neurons (Daniels et al., 2004). This is a presynaptic perturbation that results in glutamatergic vesicles of increased size (by electron microscopy) (Daniels et al., 2004). Electrophysiologically, there is a significant increase in quantal size (~ 60%). This increase in quantal size is offset by a homeostatic decrease in quantal content, and evoked responses are normal (Daniels et al., 2004).
Similar phenotypes (increased vesicle size, increased quantal size, and decreased quantal content) also result from loss-of-function mutations in synaptic vesicle endocytosis genes such as endophilin (Dickman et al., 2005; Verstreken et al., 2002), synaptojanin (Dickman et al., 2005; Verstreken et al., 2003), AP180 (Bao et al., 2005; Zhang et al., 1998), and Dap160 (Koh et al., 2004; Marie et al., 2004). Disrupted clathrin-mediated endocytosis or impaired retrieval of vesicles from bulk membrane cisternae could be responsible for the large synaptic vesicles (and hence, large quantal size). But what mechanisms are responsible for the decreases in quantal content? The answer is not entirely clear. Indeed, there are confounding issues caused by endocytic mutations, such as abnormal synaptic bouton growth (Koh et al., 2004; Marie et al., 2004; Verstreken et al., 2002), mislocalized or decreased amounts of other synaptic proteins (Bao et al., 2005; Koh et al., 2004; Marie et al., 2004; Verstreken et al., 2003; Zhang et al., 1998), and – in the case of AP180 – quantal content that is decreased so severely that evoked responses are far below normal (Bao et al., 2005). Nevertheless, several endocytic mutant NMJs have normal or only mildly diminished evoked responses to single stimuli (Koh et al., 2004; Marie et al., 2004; Verstreken et al., 2002; Verstreken et al., 2003). Therefore, as with DVGLUT overexpression, it is possible that the underlying decreases in quantal content are homeostatic in nature.
Collectively, these data suggest that the Drosophila NMJ has built-in mechanisms to downregulate presynaptic release after increases in quantal size. Yet this is not always the case. As counterexamples, increases in GluRIIA expression (relative to GluRIIB expression) and postsynaptic inhibition of PKA function cause increases in quantal size with no corresponding decreases in quantal content (Davis et al., 1998; DiAntonio et al., 1999; Petersen et al., 1997).
Questions abound. What is the difference between the increases in quantal size driven by postsynaptic GluRIIA/GluRIIB ratios and the increases in quantal size induced by DVGLUT activity or endocytosis mutations? Could the NMJ respond differently depending upon whether the original perturbation is pre- or postsynaptic? One idea that has been postulated is that glutamate in the synaptic cleft serves as a trigger that drives down presynaptic release (Daniels et al., 2004). Such a mechanism would imply that excessive cleft glutamate (or spillover glutamate) signals through glutamate receptors that are distinct from the postsynaptic GluRIIA-containing receptors. Consistent with this idea, loss of presynaptic group II metabotropic glutamate receptors at the NMJ results in increased presynaptic excitability (Howlett et al., 2008). The concept of synaptic tissues detecting “extra” neurotransmitter as a signal that eventually drives homeostatic changes is not unprecedented. For example, in mammalian hippocampal neurons, homeostatic synaptic scaling is mediated in part by glial-derived TNFα signaling, and glial detection of spillover glutamate is a key trigger of this signaling paradigm (Stellwagen and Malenka, 2006).
5. Presynaptic molecules and targets of homeostatic signaling at the NMJ
In response to postsynaptic decrease in quantal size, there is a homeostatic increase in presynaptic release at the Drosophila NMJ. How does this process work? What is the sensor that detects an “error” in the system? What is (are) the retrograde signal(s)? What are the signaling targets? In recent years, genetic approaches have been tremendously beneficial in helping to start to answer these questions. So far, many of molecules identified that are required for homeostatic signaling at the Drosophila NMJ have been placed on the presynaptic side of the synapse. They are reviewed in this section. Postsynaptic factors and retrograde signaling processes are examined in subsequent sections. A summary of many molecules discussed is in Table 1, and a collective cartoon model is depicted in Figure 2.
Table 1. Selected molecules controlling homeostatic regulation of Drosophila NMJ function.
Most molecules listed promote homeostatic increases in presynaptic release following a glutamate receptor inhibition. Gray shading indicates an opposing function: decrease in presynaptic release.
| Molecule | Tissue | Proposed Homeostatic Role | References |
|---|---|---|---|
| Cacophony(CaV2) | neuron | increase of presynaptic Ca2+ influx | (Frank et al., 2006; Frank et al., 2009; Müller and Davis, 2012) |
| Bruchpilot | neuron | increase active zone components | (Weyhersmüller et al., 2011) |
| miR-310 cluster | neuron | negative regulation of Khc-73 expression | (Tsurudome et al., 2010) |
| Khc-73 | neuron | kinesin function upstream of increased CaV2/Ca2+ influx | (Tsurudome et al., 2010) |
| Eph | neuron | signaling upstream of CaV2 | (Frank et al., 2009) |
| Ephexin | neuron | signaling upstream of CaV2 | (Frank et al., 2009) |
| Cdc42 | both | signaling upstream of CaV2 (neuron) or opposing RhoGAP Cv-c (muscle) | (Frank et al., 2009; Pilgram et al., 2011) |
| Dysbindin | neuron | presynaptic vesicle release | (Dickman and Davis, 2009) |
| Snapin | neuron | presynaptic vesicle release | (Dickman et al., 2012) |
| SNAP25 | neuron | presynaptic vesicle release | (Dickman et al., 2012) |
| Rab3-GAP | neuron | presynaptic vesicle release/RRP size | (Müller et al., 2011) |
| RIM | neuron | presynaptic RRP size regulation | (Müller et al., 2012) |
| Gooseberry | neuron | prolonged maintenance of homeostasis | (Marie et al., 2010) |
| Shal (KV4) | neuron | K+ current and Shaker inhibition | (Bergquist et al., 2010) |
| Shaker (KV1) | neuron | K+ current and Shal inhibition | (Bergquist et al., 2010) |
| Smn | neuron | prolonged maintenance of homeostasis | (Timmerman and Sanyal, 2012) |
| TOR | muscle | promotion of retrograde signaling | (Penney et al., 2012) |
| S6 kinase | muscle | promotion of retrograde signaling | (Penney et al., 2012) |
| Dystrophin | muscle | inhibition of retrograde signaling | (Pilgram et al., 2011; van der Plas et al., 2006) |
| Cv-c | muscle | inhibition of retrograde signaling | (Pilgram et al., 2011) |
| Importin 13 | muscle | inhibition of retrograde signaling | (Giagtzoglou et al., 2009) |
| CaMKII | muscle | inhibition of retrograde signaling | (Haghighi et al., 2003) |
| Gbb | muscle | retrograde BMP signaling (ligand) | (Goold and Davis, 2007; McCabe et al., 2003) |
| Wit | neuron | retrograde BMP signaling (receptor) | (Goold and Davis, 2007; Haghighi et al., 2003) |
| Mad | neuron | retrograde BMP signaling (effector) | (Goold and Davis, 2007; McCabe et al., 2003) |
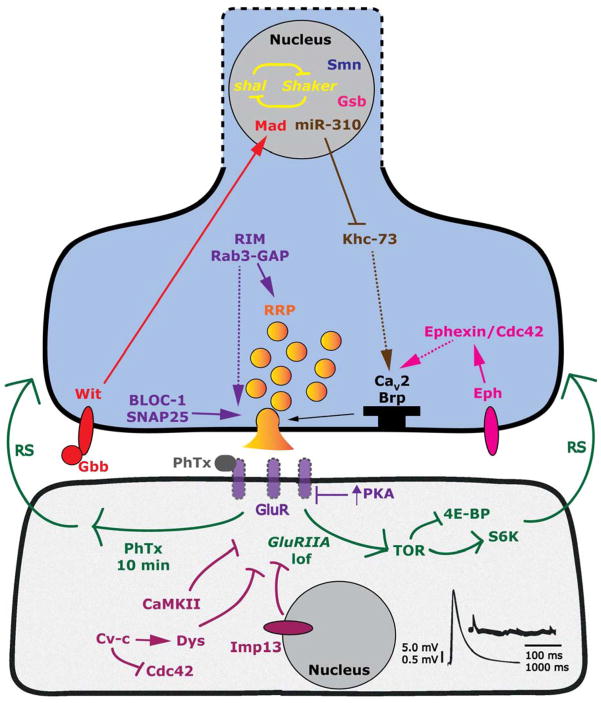
Figure 2. Model for selected molecules and processes regulating the homeostatic upregulation of presynaptic release.
Refer to text and Table 1 for additional details. Refer to Figure 1 for a simplified model. Dashed arrows represent speculative or likely signaling connections, based primarily on indirect supporting evidence.
Genetic (GluRIIA deletion) or pharmacological (PhTx) inhibition of glutamate receptor function initiates retrograde signaling processes (RS - green) that result in increased presynaptic neurotransmitter release. Postsynaptic CaMKII, Crossveinless-c (Cv-c), Dystrophin (Dys), and Importin 13 (Imp13) all oppose retrograde signaling-induced increases in presynaptic quantal content. Downstream of GluRIIA subunit receptor loss, postsynaptic Target of Rapamycin (TOR) appears to initiate a translation-dependent retrograde signaling process through S6 kinase activation (S6K). Based on genetic and electrophysiological data, some retrograde signaling may operate through Bone Morphogenetic Protein (BMP) pathway members Glass Bottom Boat (Gbb) and Wishful Thinking (Wit), or the Eph receptor tyrosine kinase (or other unknown processes).
Presynaptic proteins such as Eph/Ephexin and kinesin Khc-73 may act to potentiate presynaptic active zone (black T-bar) activity by regulating the localization or function of active zone molecules, such as CaV2-type calcium channels and the ELKS/CAST homolog, Bruchpilot (Brp). In parallel, Rab3 Interacting Molecule (RIM) and Rab3-GAP may coordinately enhance the size of the readily releasable pool of presynaptic vesicles (RRP) and also work in conjunction with members of the BLOC-1 (Dysbindin, Snapin) and SNARE (SNAP25) complexes to enhance vesicle release through SNARE-mediated fusion events.
In the presynaptic nucleus, overall neuronal excitability is shaped in part by reciprocal regulation of expression of the shal and Shaker potassium channel-encoding genes. Transcription factors Mad and Gooseberry (Gsb), as well as the Survival of Motor Neuron 1 (Smn) protein, confer competence upon the neuron to undergo homeostatic increases in synaptic release. Additionally, the miR-310 microRNA cluster negatively regulates expression of the Khc-73 gene.
CaV2-type calcium channels, enhanced calcium influx, and presynaptic active zones
Ubiquitous in metazoan nervous systems, presynaptic voltage-gated N-, P/Q-, and R-type calcium channels are critical for many types of rapid neurotransmission, and their regulation has been an intense area of study (Catterall, 2000; Currie, 2010; Dolphin, 2009; Tedford and Zamponi, 2006). Human mutations in the CaV2.1 α1 channel subunit gene CACNA1A are associated with forms of migraine and ataxia (Ophoff et al., 1996; Pietrobon, 2010; Zhuchenko et al., 1997). Since synaptic homeostasis is the process by which stable synaptic activity is maintained, an attractive idea is that this can be achieved, in part, by tight regulation of presynaptic calcium channel function. Conversely, misregulation of calcium channels could lead to neuronal instability and neurological disorders such as migraine and ataxia.
The Drosophila α1 subunit of CaV2-type calcium channels is encoded by the gene cacophony (cac) (Smith et al., 1996). Null cac mutants do not survive past the late embryonic stage of development (Kulkarni and Hall, 1987; Kurshan et al., 2009), but there are several hypomorphic, missense mutants that are viable and fertile (Brooks et al., 2003; Kawasaki et al., 2000; Smith et al., 1998); furthermore, it is possible to remove one copy of cac and have viable animals. Partial loss of cac function blocks the homeostatic increase in presynaptic release at the NMJ after glutamate receptor impairment (Frank et al., 2006; Frank et al., 2009). This result is independent of cac baseline neurotransmission defects, and it is consistent with the idea that functional, intact CaV2 channels are needed to properly upregulate neurotransmission after a homeostatic challenge (Frank et al., 2006; Frank et al., 2009).
How does this process work? This is an intense area of study. Recent work illustrates that researchers can visualize homeostatic changes at the synapse. Imaging of presynaptic Ca2+ transients during evoked stimulation shows that GluRIIA deletion (or PhTx application) induces homeostatic, presynaptic increases in calcium influx (Müller and Davis, 2012). By contrast, double mutant cacS; GluRIIA NMJs (and cacS NMJs exposed to PhTx) show no more presynaptic calcium influx than cacS mutants alone (Müller and Davis, 2012). In addition, a combination of electrophysiology and imaging has shown that PhTx application results in a rapid increase in the quantity of presynaptic active zone protein, Bruchpilot (Brp) (an ELKS/CAST homolog), an increase in the size of the presynaptic cytomatrix structure, and an increase in the size of the readily releasable pool (RRP) of presynaptic vesicles (Weyhersmüller et al., 2011). Therefore, an increase in presynaptic calcium influx, an increase in the size of the RRP, or both could facilitate increased glutamate release at the Drosophila NMJ (Figure 1B).
The miR-310 micro RNA cluster and the kinesin family member Khc-73
The above results spur another question: What are the signaling processes that control CaV2 function, enhanced presynaptic calcium influx, and enhanced RRP size? The answers are not clear, but molecules and regulatory systems that could control these aspects of synaptic homeostasis are subjects of active discovery. One such regulatory system is the miR-310 cluster of four microRNAs (miRNAs).
Genomic deletion of the miR-310 cluster results in markedly enhanced baseline NMJ neurotransmission, as well as a significant increase in the quantity of presynaptic Brp expression (Tsurudome et al., 2010). These phenotypes suggest that presynaptic calcium levels could be increased. Direct measurement of calcium transients confirms this hypothesis (Tsurudome et al., 2010). miRNAs regulate target gene expression, and in the Drosophila genome, there could be many miR-310 cluster targets. In the context of NMJ neurotransmission, the most important regulatory target appears to be the Khc-73 gene, which encodes a kinesin super family member. Partial loss of Khc-73 function can suppress the effects of miR-310 deletion, while neuronal overexpression of a Khc-73 transgene phenocopies miR-310 loss (Tsurudome et al., 2010). Importantly, miR-310 cluster overexpression and Khc-73 gene knockdown both block homeostatic compensation in response to a GluRIIA deletion (Tsurudome et al., 2010).
How is presynaptic Khc-73 exerting control over calcium influx, neurotransmission, and ultimately, synaptic homeostasis? The answer is not known, but a compelling hypothesis is that as a kinesin, Khc-73 helps to traffic synaptic active zone proteins (or other crucial synaptic proteins), resulting in the potentiation of CaV2 function (Tsurudome et al., 2010). This hypothetical function of Khc-73 could be similar to functions described for a Drosophila kinesin 3 family member, Unc-104/Imac (Barkus et al., 2008; Pack-Chung et al., 2007). There is no direct evidence that Khc-73 participates in axon transport or interacts directly with active zone components (Tsurudome et al., 2010), however, it has been shown to associate with microtubules (Kner et al., 2009).
Eph Receptor-Ephexin signaling
What other signaling processes could be important for regulating CaV2 function? Genetic and electrophysiological data suggest that a signaling system consisting of the Eph receptor tyrosine kinase, the Rho-type guanine exchange factor Ephexin, and Rho-type GTPases (Rho1, Rac1, and Cdc42) functions upstream of CaV2/Cacophony in regulating synaptic homeostasis (Frank et al., 2009). Loss of function of some of these factors alone (e.g. Ephexin) or in double heterozygous genetic combination with each other (e.g. Cdc42/+; Ephexin/+ or cac/+; Ephexin/+) results in a complete block of GluRIIA deletion-induced synaptic homeostasis (Frank et al., 2009). In the case of Ephexin mutants, this block can be partially ameliorated by overexpressing a transgenic copy of cac (Frank et al., 2009; Kawasaki et al., 2004).
As with miR-310 and Khc-73, it is unknown precisely how Ephexin and related signaling molecules might impinge upon CaV2 function. They may facilitate the trafficking of additional channels to the synapse, or they may mediate the modulation of channels already present at the synapse (Frank et al., 2009). Furthermore, Ephexin could work in conjunction with, or in parallel to, Khc-73-mediated processes. What we do know is that in other biological systems, pre- and postsynaptic Ephexin family members canonically signal through Rho-type GTPases to mediate neurite, growth cone, or dendritic spine collapse and neuromuscular development (Fu et al., 2007; Knoll and Drescher, 2004; Sahin et al., 2005; Shamah et al., 2001; Shi et al., 2010a; Shi et al., 2010b). It is intriguing to speculate that components of this conserved developmental signaling process might be co-opted by the NMJ to modulate presynaptic neurotransmitter release. Since Drosophila Eph receptor and Ephexin can bind one another, important muscle-to-nerve information may be transduced through Eph receptor tyrosine kinase (Frank et al., 2009). Notably, Ephexin is dispensable for rapid homeostatic compensation (PhTx application) but is required for long-term homeostatic compensation (GluRIIA deletion) (Frank et al., 2009). This result suggests that there are important mechanistic differences between the induction process of synaptic homeostasis and its long-term maintenance and consolidation.
BLOC-1 complex and SNARE complex members
An electrophysiology-based screen was conducted by applying PhTx to Drosophila NMJs carrying mutations in neuronally expressed genes. This screen revealed that the schizophrenia susceptibility gene dysbindin is critical for homeostatic plasticity at the Drosophila NMJ (Dickman and Davis, 2009). Dysbindin functions presynaptically, where it associates with synaptic vesicles during homeostatic compensation (Dickman and Davis, 2009). Dysbindin expression levels alter the calcium dependence of synaptic vesicle release. Yet, unlike Ephexin, genetic and electrophysiological analyses do not suggest that Dysbindin acts in conjunction with CaV2-type calcium channels in the presynaptic neuron. Rather, Dysbindin appears to act downstream or independently of calcium influx (Dickman and Davis, 2009). This information is consistent with a role for Dysbindin in modulating synaptic vesicle release probability in response to a homeostatic challenge.
Mammalian Dysbindin is a member of the BLOC-1 complex (biogenesis of lysosome-related organelles) (Starcevic and Dell’Angelica, 2004). Work from several systems suggests Dysbindin can influence many cellular processes, including synaptic vesicle biogenesis and exocytosis (Ghiani and Dell’Angelica, 2011; Ghiani et al., 2010). These facts fit well with a role for Dysbindin in the homeostatic upregulation of vesicle release. Importantly, follow-up work at the Drosophila NMJ demonstrates roles in synaptic homeostasis for Snapin (also a BLOC-1 complex member) and for SNAP25 (a component of the SNARE complex and a binding partner of Snapin) (Dickman et al., 2012). An intriguing possibility is that Dysbindin and Snapin coordinately exert control over homeostatic increases in synaptic vesicle release by modulating SNARE-mediated fusion events (Dickman et al., 2012).
Rab3-GAP, RIM, and the readily releasable vesicle pool
Additional electrophysiology-based screening uncovered an important presynaptic role for Rab3-GAP (Rab3 GTPase Activating Protein) in synaptic homeostasis (Müller et al., 2011). In mammalian systems, both Rab3 GTPase and Rab3-GAP have been implicated in regulating neurotransmission at a late stage of vesicle release (Sakane et al., 2006; Südhof, 2004). At the Drosophila NMJ, electrophysiological data show that presynaptic Rab3-GAP is required for synaptic homeostasis, and that Rab3-GAP relieves an opposing influence on homeostasis that is catalyzed by Rab3 (Müller et al., 2011). But how exactly does this process work? Could Rab3-GAP be acting at the same time point or process as Dysbindin – possibly by regulating SNARE-mediated fusion events?
New, illuminating data have emerged by examining Drosophila RIM (Rab3 interacting molecule). RIMs constitute a conserved family of proteins. Similar to roles described for its mammalian counterparts, Drosophila RIM localizes to the active zone, interacts with Rab3 GTPase molecules, and interacts with calcium channels to help them localize them to the presynaptic active zone (Graf et al., 2012; Müller et al., 2012). Beyond these similarities with mammalian RIM, Drosophila RIM is required for homeostatic compensation (Müller et al., 2012).
Given that RIM helps to localize calcium channels, a simple hypothesis would be that RIM plays some role upstream of CaV2 channels in regulating calcium influx during homeostatic compensation. Perhaps RIM constitutes a missing link between Eph/Ephexin signaling (or Khc-73 kinesin activity) and CaV2 – helping to traffic more calcium channel components to the active zone to enhance calcium influx. However, rim loss-of-function mutants have no difficulty in upregulating calcium influx in response to glutamate receptor inhibition (Müller et al., 2012). This result suggests that the role RIM plays in synaptic homeostasis is independent of its role in CaV2 regulation. It was recently demonstrated that the homeostatic increase in vesicle release is correlated with an increase in the size of the readily releasable vesicle pool (RRP) (Müller et al., 2012; Weyhersmüller et al., 2011). Follow-up electrophysiological experiments utilizing high frequency stimulation and EPSC amplitude fluctuation analysis point to a role for RIM in regulating the size of the RRP during homeostatic compensation (Müller et al., 2012). Finally, genetic data suggest that RIM could be performing this function in conjunction with Rab3-GAP (Müller et al., 2012).
Gooseberry transcription factor
Gooseberry (Gsb) – a pair-rule transcription factor – was identified as an essential component of GluRIIA mutant-induced synaptic homeostatic compensation (Marie et al., 2010). Gsb has long been known to be important for embryonic neuroblast determination (Skeath et al., 1995), but it is also expressed in mature motor neurons (Marie et al., 2010). Like Ephexin, Gsb is dispensable for the rapid induction of synaptic homeostasis (Marie et al., 2010), demonstrating once again that it is possible to uncouple the short-term induction of homeostatic increases in presynaptic release from the long-term maintenance of those increases.
Interestingly, Gsb assumes an opposing role with Wnt/Wingless (Wg) signaling during embryonic cell fate specification (Bhat et al., 2000; Duman-Scheel et al., 1997). Genetic and electrophysiological data suggest that this antagonistic relationship is maintained during synaptic homeostasis (Marie et al., 2010). In the context of the NMJ, this is an especially intriguing result because Wnt/Wg signaling also plays important roles in NMJ development (Korkut et al., 2009; Mathew et al., 2005; Miech et al., 2008). This underscores possible links between NMJ developmental processes and long-term maintenance of synapse function.
Potassium channels and a hierarchy of homeostatic signals
An additional protein that emerged from searches for homeostatic factors is Drosophila Shal (Shaker cognate I). Shal is a KV4-type potassium channel protein, which along with Drosophila Shaker (KV1) mediates potassium current in the neuronal cell body. shal loss-of-function mutants have completely impaired homeostatic responses to PhTx or GluRIIA mutant challenges (Bergquist et al., 2010).
At first glance, this result is puzzling. One would expect the loss of a potassium channel to enhance the excitability of a cell – and possibly to drive a robust increase in transmitter release. However, in this particular case, quantitative RT-PCR experiments show that shal and Shaker gene function and expression are inversely coupled (possibly due to a separate homeostatic maintenance of potassium current). Decreased shal gene function leads to increased Shaker gene expression and vice versa (Bergquist et al., 2010). As a result, the increased Shaker protein expressed in shal mutants completely impairs the cell’s ability to respond to homeostatic challenges. Simple overexpression of Shaker has the same effect: silencing of homeostatic compensation (Bergquist et al., 2010).
Why does overexpression of Shaker block synaptic homeostasis? Channel localization may be the answer. Shal channels are restricted to the cell body (Bergquist et al., 2010; Tsunoda and Salkoff, 1995), whereas Shaker can localize to the synaptic terminal (Ganetzky and Wu, 1982; Jan et al., 1977; Wu et al., 1983). NMJ blockade of Shaker potassium channels using 4-aminopyradine successfully restores homeostatic responses in shal mutants (Bergquist et al., 2010). Taken all together, these results highlight the importance of a neuron’s overall excitability in conferring competence to respond to a homeostatic challenge.
Survival Motor Neuron 1
An intriguing link has been made between synaptic homeostasis and the Drosophila homolog of Survival Motor Neuron 1 (Smn) protein. Smn family members were previously shown to regulate small nuclear ribonucleoprotein (snRNP) biogenesis (Fischer et al., 1997), and loss of human SMN1 or SMN2 function causes spinal muscular atrophy (Coovert et al., 1997; Lefebvre et al., 1997). Several studies of Smn in invertebrate model systems have been conducted to help elucidate the mechanisms by which it acts and to generate invertebrate genetic models of spinal muscular atrophy (Briese et al., 2009; Chan et al., 2003; Chang et al., 2008; Rajendra et al., 2007; Sleigh et al., 2011).
By taking advantage of RNA interference (RNAi)-based tools and tissue specific gene knockdown, it was recently demonstrated that presynaptic Drosophila Smn is required for long-term homeostatic compensation (Timmerman and Sanyal, 2012). Yet, as with Ephexin and Gsb, Smn is dispensable for rapid homeostatic compensation (Timmerman and Sanyal, 2012). These new results raise the intriguing possibility that regulation of small nuclear RNAs or mRNA metabolism could be involved in consolidating long-term homeostatic responses. They also raise possible connections between homeostatic plasticity and disorders like spinal muscular atrophy.
6. Postsynaptic molecules regulating retrograde increases in presynaptic release
The muscle-to-nerve signals regulated by the proteins reviewed in this section increase quantal content, even in the absence of a homeostatic challenge, like GluRIIA deletion. These proteins may represent core components (or in some cases, negative regulators) of postsynaptic signaling processes downstream of glutamate receptor inhibition.
Target of Rapamycin
One factor in the muscle that propagates a retrograde signal in order to increase presynaptic release is Drosophila Target of Rapamycin (TOR) (Penney et al., 2012). Postsynaptic overexpression of TOR – or postsynaptic expression of a constitutively active TOR target, S6 Kinase (S6K) – induces increases in presynaptic quantal content (Penney et al., 2012). These results suggest that TOR is capable of propagating an instructive signal through S6K to increase presynaptic neurotransmitter release. Is this TOR-induced signal activated when postsynaptic GluRIIA-containing receptors are absent? It appears that it is. Homeostatic compensation is completely blocked in GluRIIA; Tor double loss-of-function mutants. Furthermore, when TOR is overexpressed postsynaptically in a GluRIIA mutant background, there is no additive effect on release – i.e., there is no further increase in quantal content compared to GluRIIA mutants alone (Penney et al., 2012). Collectively, these results suggest that GluRIIA mutant-induced forms of synaptic homeostasis proceed through TOR signaling.
Canonically, TOR promotes protein translation – either by phosphorylating S6K, which promotes cap-dependent translation (Holz et al., 2005; Ma and Blenis, 2009), or by inhibiting the activity of the eIF4E binding protein, 4E-BP (Gingras et al., 2001; Sonenberg and Hinnebusch, 2009). At the Drosophila NMJ, could a similar promotion of protein translation result in the production of factors needed for synaptic homeostasis? It appears so. Not only does S6K activation promote enhanced presynaptic release, but also, transgenic expression of a TOR-independent form of 4E-BP in the muscle impairs homeostatic compensation (Penney et al., 2012). Superficially, these results appear to be in conflict with prior data that rapid homeostatic compensation at the NMJ does not require protein translation (Frank et al., 2006). However, in the present case, the authors were not examining PhTx-induced compensation; instead they were examining GluIIA loss-induced compensation. As stated before, it is likely that distinct (or partially overlapping) mechanisms are required for different forms of homeostatic compensation: in this case, rapid, protein translation-independent homeostatic plasticity (PhTx), and long-term, protein translation-dependent homeostatic plasticity (GluRIIA).
The fact that Drosophila TOR is required for NMJ homeostasis matches nicely with the fact that TOR has been implicated in forms of synaptic plasticity in mammalian systems (Hoeffer and Klann, 2010; Jaworski and Sheng, 2006). Of particular interest, mammalian Target of Rapamycin (mTOR) has recently been shown to participate in a retrograde form of homeostatic plasticity that results in increased presynaptic release (Henry et al., 2012). This signaling process is gated (at least in part) by presynaptic calcium channel function (Jakawich et al., 2010). These findings mirror salient features of retrograde homeostatic signaling at the Drosophila NMJ (Frank et al., 2006; Penney et al., 2012) and raise the possibility for ancient roles in homeostatic plasticity for factors like TOR and presynaptic calcium channels. However, there are important distinctions. Perhaps most notably, the retrograde signaling system in mammalian neurons proceeds through release of Brain-Derived Neurotrophic Factor (BDNF) (Henry et al., 2012). BDNF and its canonical TrkB receptors are absent from Drosophila.
Dystrophin and Crossveinless-c
Drosophila TOR promotes increased presynaptic release at the NMJ. Loss of TOR blocks GluRIIA deletion-induced homeostatic compensation. Are there postsynaptic factors that behave in the opposite way – factors whose loss leads to increased presynaptic release and whose activity could be opposing TOR? The answer is yes; three Drosophila proteins that fit this description are Dystrophin, the RhoGAP Crossveinless-c (Cv-c), and Importin 13 (Imp13).
Human dystrophin mutations cause muscular dystrophy (Hoffman et al., 1987), Dystrophin functions to couple muscle cytoskeleton to the extracellular matrix, which helps to maintain muscle strength and integrity. In addition, Dystrophin interacts with intracellular signaling molecules (Rando, 2001). Genetic model studies of Dystrophin have sought to elucidate how it might regulate signaling processes relevant to disease.
At the Drosophila NMJ, large isoforms of muscle Dystrophin localize at synaptic and extrasynaptic sites (van der Plas et al., 2006). In dystrophin mutants that lack these isoforms, NMJ development appears completely normal (van der Plas et al., 2006). By electrophysiology, dystrophin mutant NMJ quantal size is normal, but quantal content is significantly increased, resulting in enhanced evoked amplitudes (van der Plas et al., 2006). Postsynaptic Dystrophin rescues this electrophysiological phenotype. Therefore, postsynaptic Dystrophin downregulates presynaptic neurotransmitter release, the opposite result of what has been documented for TOR.
A genetic screen found that cv-c loss-of-function mutations interact genetically with dystrophin mutations in Drosophila wing cross vein formation (Pilgram et al., 2011). Examination of the dystrophin/cv-c genetic relationship at the NMJ showed that postsynaptic loss of cv-c phenocopies dystrophin loss (normal quantal size, and significantly increased quantal content) (Pilgram et al., 2011). Dystrophin appears to act downstream of Cv-c in the muscle. Postsynaptic overexpression of dystrophin partially suppresses the enhanced quantal content phenotype of cv-c mutants. By contrast, postsynaptic expression of transgenic cv-c does not suppress enhanced quantal content of dystrophin mutants (Pilgram et al., 2011).
As a RhoGAP, Cv-c negatively regulates Rho-type GTPase function. Interestingly, Rho-type GTPase Cdc42 loss-of-function mutations partially suppress cv-c mutations (Pilgram et al., 2011). Together with studies of the RhoGEF Ephexin, this result potentially places Cdc42 function at both pre- and postsynaptic sites in the execution of retrograde homeostatic signaling (Frank et al., 2009; Pilgram et al., 2011).
Importin 13
Importin receptors facilitate transport through nuclear pore complexes. In Drosophila, Imp13 localizes to nuclei in both neurons and muscles (Giagtzoglou et al., 2009). Electrophysiologically, imp13 loss-of-function mutations phenocopy dystrophin and cv-c losses of function: normal quantal size, increased quantal content (Giagtzoglou et al., 2009). Expressing transgenic imp13 in the neurons fails to rescue the enhanced release phenotype, but expressing it in the muscle does rescue (Giagtzoglou et al., 2009). The electrophysiology is corroborated by presynaptic calcium imaging experiments showing increased levels of presynaptic intracellular calcium in imp13 mutants; this phenotype is also rescued by postsynaptic expression of transgenic imp13 (Giagtzoglou et al., 2009).
Since loss of postsynaptic imp13 leads to presynaptic increases in quantal content, a simple model could be that Imp13 somehow controls the expression of the glutamate receptors, which results in retrograde control of presynaptic release. However, quantal size is not affected in imp13 mutants, and neither are expression levels of GluRIIA or GluRIIB subunits (Giagtzoglou et al., 2009). Currently, it is unknown how Imp13 may interact with Dystrophin, Cv-c, or, TOR function in the muscle, or even if imp13 loss of function combined with TOR gain of function has additive or non-additive effects on presynaptic release. Future experiments along these lines could help illustrate how retrograde homeostatic signaling processes take shape in the muscle.
7. What are the retrograde homeostatic signals at the NMJ
It has been clear for a long while that retrograde signaling mechanisms are important for synaptic homeostasis at the NMJ. To date, however, the precise, instructive retrograde signals required for synaptic homeostasis are elusive.
Evidence against canonical retrograde signaling
Retrograde signals abound in mammalian neuronal systems such as endocanabinoids, Brain-Derived Neurotrophic Factor (BDNF), and nitric oxide (NO). Yet these signals do not appear to constitute retrograde homeostatic signals at the Drosophila NMJ. Neither orthologs of cannabinoid receptors, nor BDNF, nor the BDNF TrkB receptor are found in Drosophila. NO is a prominent signaling molecule in insects, but at the Drosophila NMJ, evidence suggests that NO originates in the presynaptic nerve, rather than the postsynaptic muscle. Application of NO donors to the NMJ results in cyclic GMP immunoreactivity presynaptically, but not postsynaptically (Wildemann and Bicker, 1999a, b).
Another prominent retrograde signaling molecule at the Drosophila NMJ is Synaptotagmin 4 (Syt4). Syt4 is reported to act as a postsynaptic calcium sensor that induces a retrograde signaling process controlling spontaneous miniature activity, evoked neurotransmission, and local synapse development (Barber et al., 2009; Yoshihara et al., 2005). The effects on neurotransmission make Syt4 an excellent candidate for controlling synaptic homeostasis. Drastic changes in the frequency of spontaneous release could reflect relevant changes to the presynaptic RRP or release probability. However, a direct test of Syt4 mutant NMJs for a role in rapid homeostatic compensation did not reveal one (Dickman and Davis, 2009), and to date, it is unclear what (if any) role Syt4 might play in the homeostatic regulation of synapse function.
Bone Morphogenetic Protein signaling
One retrograde system that is required for NMJ homeostasis is Bone Morphogenetic Protein (BMP) signaling. Together, a pair of studies has convincingly demonstrated that the muscle-derived BMP ligand Glass Bottom Boat (Gbb) and its presynaptic receptor Wishful Thinking (Wit) are needed for both rapid (PhTx) and long-term (GluRIIA mutant) forms of synaptic homeostasis (Goold and Davis, 2007; Haghighi et al., 2003). Presynaptic Wit appears to act through retrograde axonal transport to activate the Drosophila Mad (Smad) transcription factor (McCabe et al., 2003), and this event is necessary for PhTx-induced synaptic homeostasis (Goold and Davis, 2007). In addition, BMP signaling is required for increases in presynaptic release that are driven either by postsynaptic calcium/calmodulin-dependent protein kinase II (CaMKII) inhibition (Haghighi et al., 2003) or by dystrophin mutation (van der Plas et al., 2006). Taken together, these results ideally position BMP signaling to propagate retrograde information to induce increased neurotransmitter release.
Yet current data do not support an instructive role for BMP/Mad signaling during rapid homeostatic compensation. Rather, BMP/Mad function appears permissive. First, severing the segmental motor axons – hence eliminating retrograde transport to the nucleus – does not impair PhTx-induced increases in quantal content (Frank et al., 2006). Second, inhibition of BMP/Mad signaling at various points in developmental time demonstrates a requirement for signaling well before PhTx application (Goold and Davis, 2007). This latter result was achieved by exploiting the P[Switch] expression system, which allows Drosophila researchers exquisite control over both the spatial and temporal expression of target UAS transgenes (Osterwalder et al., 2001; Roman et al., 2001). Using this system, expression of a transgenic Mad inhibitor, UAS-dad, blocks synaptic homeostasis. The longer during development that UAS-dad is expressed, the more severe the block (Goold and Davis, 2007). One day of UAS-dad expression leaves rapid, PhTx-induced homeostatic compensation largely in tact, while longer periods of UAS-dad expression render a severe block (Goold and Davis, 2007).
At this juncture, it may be premature to conclude that BMP/Mad signaling plays no instructive role during homeostatic compensation at the NMJ. The above results point to a permissive role for BMP/Mad during PhTx-induced homeostatic compensation (Frank et al., 2006; Goold and Davis, 2007). Yet it is quite possible BMP/Mad signaling instructively consolidates long-term homeostatic changes that are needed after GluRIIA loss. Furthermore, acute removal of Gbb or Wit (which could test for instructive signaling roles for the BMP ligand and receptor) has not been attempted. Therefore, there exists a formal possibility of instructive, non-Mad-mediated BMP signaling during rapid synaptic homeostasis.
8. Concluding Remarks
The Drosophila NMJ compared with vertebrate models of homeostatic synaptic plasticity
As detailed above, the Drosophila NMJ shares several aspects of homeostatic plasticity with vertebrate central synapses. Retrograde signaling driven by postsynaptic TOR function is prominent for both the Drosophila NMJ and hippocampal synapses (Henry et al., 2012; Penney et al., 2012). The requirement of presynaptic calcium channel function is also an important similarity (Frank et al., 2006; Frank et al., 2009; Jakawich et al., 2010), as is the demonstration that challenges to synaptic function result in increases in presynaptic calcium influx (Müller and Davis, 2012; Zhao et al., 2011). There is also evidence that spontaneous miniature synaptic events shape the time course and robustness of homeostatic responses in Drosophila and mammalian systems (Frank et al., 2006; Sutton et al., 2006).
But how do the ultimate homeostatic expression systems compare between insect and vertebrate synapses? Homeostatic signaling at the Drosophila NMJ redounds to changes in presynaptic quantal content. This fact appears to represent an important difference between the Drosophila NMJ and hippocampal synapses that employ postsynaptic scaling mechanisms to globally strengthen or weaken synaptic efficacy via AMPA-type glutamate receptor addition or subtraction (O’Brien et al., 1998; Turrigiano et al., 1998). However, depending upon the preparation used or the manipulation employed, hippocampal synapses also display homeostatic changes in presynaptic release properties and RRP size, not unlike the Drosophila NMJ (Burrone et al., 2002; Murthy et al., 2001; Thiagarajan et al., 2005).
Concerning peripheral synapses, fascinating similarities exist between the glutamatergic Drosophila NMJ and the cholinergic mammalian NMJ. For example, in the human neuromuscular disorder myasthenia gravis, acetylcholine receptor function is impaired. Electrophysiological recordings show that myasthenic muscle endplates have dramatically reduced quantal size, coupled with increased quantal content (Cull-Candy et al., 1980; Plomp et al., 1995). The same is also true for a rat model of myasthenia gravis induced by α-bungarotoxin (Plomp et al., 1995; Plomp et al., 1992, 1994). At a basic level of homeostatic compensation, these results are strikingly reminiscent of the Drosophila NMJ model.
Challenges for the future
Moving forward, there are several important challenges to address at the Drosophila NMJ. An obvious goal is to identify and characterize the retrograde muscle-to-nerve signals that result in homeostatic increases (or decreases) of neurotransmitter release. It is also necessary to conduct further characterization of the molecules already identified that participate in synaptic homeostasis (Table 1). Which ones act upstream of CaV2 to control levels of presynaptic calcium influx? How do they do it? Which ones act upstream or in conjunction with RIM to control RRP size? How do the known molecules connect with each other to form coherent signaling pathways?
A major question for any molecule implicated in synaptic homeostasis is whether it instructively drives homeostatic adjustments in quantal content or whether it permissively guides the synapse to be competent to respond to homeostatic challenges. To help address this problem, there are many tools that Drosophilists can use to acutely add or subtract homeostatic factors from the synapse such as RNAi-based transgenic constructs (Dietzl et al., 2007), the aforementioned P[Switch] expression system (Osterwalder et al., 2001; Roman et al., 2001), protein photoinactivation techniques like FlAsH-FALI (Heerssen et al., 2008; Marek and Davis, 2002; Poskanzer et al., 2003), and traditional pharmacology. It is tempting to assume that molecules required for the rapid, PhTx-induced form of synaptic homeostasis are more likely to represent instructive signals, while molecules required for the sustained, GluRIIA mutant-induced form are more likely to represent permissive ones. Yet neither assumption is necessarily true. It is possible that some molecules – like BMP-induced Mad – are required to provide a developmental framework to allow the rapid induction of synaptic homeostasis. Conversely, it is possible that other molecules are required for continuous, active signaling during the long-term maintenance of synaptic homeostasis.
Clearly, the Drosophila NMJ is a genetically tractable system. But ultimately, will this insect model help us to identify molecules and signaling processes that are important for neuronal stability in mammalian central and peripheral nervous systems? Is the NMJ home to important universal signaling processes that direct synaptic stability? Will these signaling processes prove relevant to human health and physiology? It is extremely likely. The list of Drosophila proteins implicated in synaptic homeostasis at the NMJ includes several that have human homologs with roles in neurological or muscular disorders such as: ataxia and migraine (CaV2.1/Cacophony) (Adams and Snutch, 2007; Pietrobon, 2010); schizophrenia (Dysbindin) (Ross et al., 2006); pain, epilepsy, and heart arrhythmia (KV4/Shal) (Birnbaum et al., 2004; Castro et al., 2001); spinal muscular atrophy (Smn) (Coovert et al., 1997; Lefebvre et al., 1997); and muscular dystrophy (Dystrophin) (Hoffman et al., 1987). It is entirely possible that some of these conditions are caused or exacerbated by underlying defects in synaptic homeostasis. Thus, basic molecular information uncovered about the homeostatic regulation of Drosophila NMJ function may be relevant to human neurological disorders.
Highlights.
A summary of homeostatic plasticity at the Drosophila neuromuscular junction (NMJ)
Electrophysiological results demonstrating homeostatic control of NMJ function
Current understanding of molecular mechanisms that drive synaptic homeostasis
Presynaptic and postsynaptic molecules controlling NMJ homeostasis
Notable connections with other experimental systems
Acknowledgments
Many thanks to Dion Dickman, Martin Müller, Douglas Brusich, and anonymous reviewers for helpful comments on text and figures. CAF’s laboratory is supported in part by NIH R00 Award NS062738, as well as funds from the Department of Anatomy and Cell Biology at the University of Iowa Carver College of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams PJ, Snutch TP. Calcium channelopathies: voltage-gated calcium channels. Subcell Biochem. 2007;45:215–251. doi: 10.1007/978-1-4020-6191-2_8. [DOI] [PubMed] [Google Scholar]
- Albin SD, Davis GW. Coordinating structural and functional synapse development: postsynaptic p21-activated kinase independently specifies glutamate receptor abundance and postsynaptic morphology. J Neurosci. 2004;24:6871–6879. doi: 10.1523/JNEUROSCI.1538-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao H, Daniels RW, MacLeod GT, Charlton MP, Atwood HL, Zhang B. AP180 maintains the distribution of synaptic and vesicle proteins in the nerve terminal and indirectly regulates the efficacy of Ca2+-triggered exocytosis. J Neurophysiol. 2005;94:1888–1903. doi: 10.1152/jn.00080.2005. [DOI] [PubMed] [Google Scholar]
- Barber CF, Jorquera RA, Melom JE, Littleton JT. Postsynaptic regulation of synaptic plasticity by synaptotagmin 4 requires both C2 domains. J Cell Biol. 2009;187:295–310. doi: 10.1083/jcb.200903098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkus RV, Klyachko O, Horiuchi D, Dickson BJ, Saxton WM. Identification of an axonal kinesin-3 motor for fast anterograde vesicle transport that facilitates retrograde transport of neuropeptides. Mol Biol Cell. 2008;19:274–283. doi: 10.1091/mbc.E07-03-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergquist S, Dickman DK, Davis GW. A hierarchy of cell intrinsic and target-derived homeostatic signaling. Neuron. 2010;66:220–234. doi: 10.1016/j.neuron.2010.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat KM, van Beers EH, Bhat P. Sloppy paired acts as the downstream target of wingless in the Drosophila CNS and interaction between sloppy paired and gooseberry inhibits sloppy paired during neurogenesis. Development. 2000;127:655–665. doi: 10.1242/dev.127.3.655. [DOI] [PubMed] [Google Scholar]
- Birnbaum SG, Varga AW, Yuan LL, Anderson AE, Sweatt JD, Schrader LA. Structure and function of Kv4-family transient potassium channels. Physiol Rev. 2004;84:803–833. doi: 10.1152/physrev.00039.2003. [DOI] [PubMed] [Google Scholar]
- Briese M, Esmaeili B, Fraboulet S, Burt EC, Christodoulou S, Towers PR, Davies KE, Sattelle DB. Deletion of smn-1, the Caenorhabditis elegans ortholog of the spinal muscular atrophy gene, results in locomotor dysfunction and reduced lifespan. Hum Mol Genet. 2009;18:97–104. doi: 10.1093/hmg/ddn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadie KS, Bate M. Development of the embryonic neuromuscular synapse of Drosophila melanogaster. J Neurosci. 1993;13:144–166. doi: 10.1523/JNEUROSCI.13-01-00144.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks IM, Felling R, Kawasaki F, Ordway RW. Genetic analysis of a synaptic calcium channel in Drosophila: intragenic modifiers of a temperature-sensitive paralytic mutant of cacophony. Genetics. 2003;164:163–171. doi: 10.1093/genetics/164.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrone J, O’Byrne M, Murthy VN. Multiple forms of synaptic plasticity triggered by selective suppression of activity in individual neurons. Nature. 2002;420:414–418. doi: 10.1038/nature01242. [DOI] [PubMed] [Google Scholar]
- Castro PA, Cooper EC, Lowenstein DH, Baraban SC. Hippocampal heterotopia lack functional Kv4.2 potassium channels in the methylazoxymethanol model of cortical malformations and epilepsy. J Neurosci. 2001;21:6626–6634. doi: 10.1523/JNEUROSCI.21-17-06626.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA. Structure and regulation of voltage-gated Ca2+ channels. Annu Rev Cell Dev Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- Chan YB, Miguel-Aliaga I, Franks C, Thomas N, Trulzsch B, Sattelle DB, Davies KE, van den Heuvel M. Neuromuscular defects in a Drosophila survival motor neuron gene mutant. Hum Mol Genet. 2003;12:1367–1376. doi: 10.1093/hmg/ddg157. [DOI] [PubMed] [Google Scholar]
- Chang HC, Dimlich DN, Yokokura T, Mukherjee A, Kankel MW, Sen A, Sridhar V, Fulga TA, Hart AC, Van Vactor D, et al. Modeling spinal muscular atrophy in Drosophila. PLoS One. 2008;3:e3209. doi: 10.1371/journal.pone.0003209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coovert DD, Le TT, McAndrew PE, Strasswimmer J, Crawford TO, Mendell JR, Coulson SE, Androphy EJ, Prior TW, Burghes AH. The survival motor neuron protein in spinal muscular atrophy. Hum Mol Genet. 1997;6:1205–1214. doi: 10.1093/hmg/6.8.1205. [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Miledi R, Uchitel OD. Diffusion of acetylcholine in the synaptic cleft of normal and myasthenia gravis human endplates. Nature. 1980;286:500–502. doi: 10.1038/286500a0. [DOI] [PubMed] [Google Scholar]
- Currie KP. G protein modulation of CaV2 voltage-gated calcium channels. Channels (Austin) 2010;4:497–509. doi: 10.4161/chan.4.6.12871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels RW, Collins CA, Gelfand MV, Dant J, Brooks ES, Krantz DE, DiAntonio A. Increased expression of the Drosophila vesicular glutamate transporter leads to excess glutamate release and a compensatory decrease in quantal content. J Neurosci. 2004;24:10466–10474. doi: 10.1523/JNEUROSCI.3001-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis GW. Homeostatic control of neural activity: from phenomenology to molecular design. Annu Rev Neurosci. 2006;29:307–323. doi: 10.1146/annurev.neuro.28.061604.135751. [DOI] [PubMed] [Google Scholar]
- Davis GW, DiAntonio A, Petersen SA, Goodman CS. Postsynaptic PKA controls quantal size and reveals a retrograde signal that regulates presynaptic transmitter release in Drosophila. Neuron. 1998;20:305–315. doi: 10.1016/s0896-6273(00)80458-4. [DOI] [PubMed] [Google Scholar]
- Davis GW, Goodman CS. Synapse-specific control of synaptic efficacy at the terminals of a single neuron. Nature. 1998;392:82–86. doi: 10.1038/32176. [DOI] [PubMed] [Google Scholar]
- Davis GW, Schuster CM, Goodman CS. Genetic analysis of the mechanisms controlling target selection: target-derived Fasciclin II regulates the pattern of synapse formation. Neuron. 1997;19:561–573. doi: 10.1016/s0896-6273(00)80372-4. [DOI] [PubMed] [Google Scholar]
- DiAntonio A, Petersen SA, Heckmann M, Goodman CS. Glutamate receptor expression regulates quantal size and quantal content at the Drosophila neuromuscular junction. J Neurosci. 1999;19:3023–3032. doi: 10.1523/JNEUROSCI.19-08-03023.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickman DK, Davis GW. The schizophrenia susceptibility gene dysbindin controls synaptic homeostasis. Science. 2009;326:1127–1130. doi: 10.1126/science.1179685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickman DK, Horne JA, Meinertzhagen IA, Schwarz TL. A slowed classical pathway rather than kiss-and-run mediates endocytosis at synapses lacking synaptojanin and endophilin. Cell. 2005;123:521–533. doi: 10.1016/j.cell.2005.09.026. [DOI] [PubMed] [Google Scholar]
- Dickman DK, Tong A, Davis GW. Snapin is critical for presynaptic homeostatic plasticity. J Neurosci. 2012;32:8716–8724. doi: 10.1523/JNEUROSCI.5465-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- Dolphin AC. Calcium channel diversity: multiple roles of calcium channel subunits. Curr Opin Neurobiol. 2009;19:237–244. doi: 10.1016/j.conb.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Duman-Scheel M, Li X, Orlov I, Noll M, Patel NH. Genetic separation of the neural and cuticular patterning functions of gooseberry. Development. 1997;124:2855–2865. doi: 10.1242/dev.124.15.2855. [DOI] [PubMed] [Google Scholar]
- Eldefrawi AT, Eldefrawi ME, Konno K, Mansour NA, Nakanishi K, Oltz E, Usherwood PN. Structure and synthesis of a potent glutamate receptor antagonist in wasp venom. Proc Natl Acad Sci U S A. 1988;85:4910–4913. doi: 10.1073/pnas.85.13.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer U, Liu Q, Dreyfuss G. The SMN-SIP1 complex has an essential role in spliceosomal snRNP biogenesis. Cell. 1997;90:1023–1029. doi: 10.1016/s0092-8674(00)80368-2. [DOI] [PubMed] [Google Scholar]
- Frank CA, Kennedy MJ, Goold CP, Marek KW, Davis GW. Mechanisms underlying the rapid induction and sustained expression of synaptic homeostasis. Neuron. 2006;52:663–677. doi: 10.1016/j.neuron.2006.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank CA, Pielage J, Davis GW. A presynaptic homeostatic signaling system composed of the Eph receptor, ephexin, Cdc42, and CaV2.1 calcium channels. Neuron. 2009;61:556–569. doi: 10.1016/j.neuron.2008.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu WY, Chen Y, Sahin M, Zhao XS, Shi L, Bikoff JB, Lai KO, Yung WH, Fu AK, Greenberg ME, et al. Cdk5 regulates EphA4-mediated dendritic spine retraction through an ephexin1-dependent mechanism. Nat Neurosci. 2007;10:67–76. doi: 10.1038/nn1811. [DOI] [PubMed] [Google Scholar]
- Ganetzky B, Wu CF. Drosophila mutants with opposing effects on nerve excitability: genetic and spatial interactions in repetitive firing. J Neurophysiol. 1982;47:501–514. doi: 10.1152/jn.1982.47.3.501. [DOI] [PubMed] [Google Scholar]
- Ghiani CA, Dell’Angelica EC. Dysbindin-containing complexes and their proposed functions in brain: from zero to (too) many in a decade. ASN Neuro. 2011;3 doi: 10.1042/AN20110010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiani CA, Starcevic M, Rodriguez-Fernandez IA, Nazarian R, Cheli VT, Chan LN, Malvar JS, de Vellis J, Sabatti C, Dell’Angelica EC. The dysbindin-containing complex (BLOC-1) in brain: developmental regulation, interaction with SNARE proteins and role in neurite outgrowth. Mol Psychiatry. 2010;15(115):204–115. doi: 10.1038/mp.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giagtzoglou N, Lin YQ, Haueter C, Bellen HJ. Importin 13 regulates neurotransmitter release at the Drosophila neuromuscular junction. J Neurosci. 2009;29:5628–5639. doi: 10.1523/JNEUROSCI.0794-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras AC, Raught B, Sonenberg N. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 2001;15:807–826. doi: 10.1101/gad.887201. [DOI] [PubMed] [Google Scholar]
- Goold CP, Davis GW. The BMP ligand Gbb gates the expression of synaptic homeostasis independent of synaptic growth control. Neuron. 2007;56:109–123. doi: 10.1016/j.neuron.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf ER, Valakh V, Wright CM, Wu C, Liu Z, Zhang YQ, Diantonio A. RIM Promotes Calcium Channel Accumulation at Active Zones of the Drosophila Neuromuscular Junction. J Neurosci. 2012;32:16586–16596. doi: 10.1523/JNEUROSCI.0965-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenningloh G, Rehm EJ, Goodman CS. Genetic analysis of growth cone guidance in Drosophila: fasciclin II functions as a neuronal recognition molecule. Cell. 1991;67:45–57. doi: 10.1016/0092-8674(91)90571-f. [DOI] [PubMed] [Google Scholar]
- Haghighi AP, McCabe BD, Fetter RD, Palmer JE, Hom S, Goodman CS. Retrograde control of synaptic transmission by postsynaptic CaMKII at the Drosophila neuromuscular junction. Neuron. 2003;39:255–267. doi: 10.1016/s0896-6273(03)00427-6. [DOI] [PubMed] [Google Scholar]
- Heckscher ES, Fetter RD, Marek KW, Albin SD, Davis GW. NF-kappaB, IkappaB, and IRAK control glutamate receptor density at the Drosophila NMJ. Neuron. 2007;55:859–873. doi: 10.1016/j.neuron.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerssen H, Fetter RD, Davis GW. Clathrin dependence of synaptic-vesicle formation at the Drosophila neuromuscular junction. Curr Biol. 2008;18:401–409. doi: 10.1016/j.cub.2008.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry FE, McCartney AJ, Neely R, Perez AS, Carruthers CJ, Stuenkel EL, Inoki K, Sutton MA. Retrograde changes in presynaptic function driven by dendritic mTORC1. J Neurosci. 2012;32:17128–17142. doi: 10.1523/JNEUROSCI.2149-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33:67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Holz MK, Ballif BA, Gygi SP, Blenis J. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell. 2005;123:569–580. doi: 10.1016/j.cell.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Howlett E, Lin CC, Lavery W, Stern M. A PI3-kinase-mediated negative feedback regulates neuronal excitability. PLoS Genet. 2008;4:e1000277. doi: 10.1371/journal.pgen.1000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson H, Usherwood PN. Spider toxins as tools for dissecting elements of excitatory amino acid transmission. Trends Neurosci. 1988;11:278–283. doi: 10.1016/0166-2236(88)90112-9. [DOI] [PubMed] [Google Scholar]
- Jakawich SK, Nasser HB, Strong MJ, McCartney AJ, Perez AS, Rakesh N, Carruthers CJ, Sutton MA. Local presynaptic activity gates homeostatic changes in presynaptic function driven by dendritic BDNF synthesis. Neuron. 2010;68:1143–1158. doi: 10.1016/j.neuron.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan LY, Jan YN. L-glutamate as an excitatory transmitter at the Drosophila larval neuromuscular junction. J Physiol. 1976a;262:215–236. doi: 10.1113/jphysiol.1976.sp011593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan LY, Jan YN. Properties of the larval neuromuscular junction in Drosophila melanogaster. J Physiol. 1976b;262:189–214. doi: 10.1113/jphysiol.1976.sp011592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan YN, Jan LY, Dennis MJ. Two mutations of synaptic transmission in Drosophila. Proc R Soc Lond B Biol Sci. 1977;198:87–108. doi: 10.1098/rspb.1977.0087. [DOI] [PubMed] [Google Scholar]
- Jarecki J, Keshishian H. Role of neural activity during synaptogenesis in Drosophila. J Neurosci. 1995;15:8177–8190. doi: 10.1523/JNEUROSCI.15-12-08177.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski J, Sheng M. The growing role of mTOR in neuronal development and plasticity. Mol Neurobiol. 2006;34:205–219. doi: 10.1385/MN:34:3:205. [DOI] [PubMed] [Google Scholar]
- Karst H, Piek T. Structure-activity relationship of philanthotoxins--II. Effects on the glutamate gated ion channels of the locust muscle fibre membrane. Comp Biochem Physiol C. 1991;98:479–489. doi: 10.1016/0742-8413(91)90237-n. [DOI] [PubMed] [Google Scholar]
- Kawasaki F, Felling R, Ordway RW. A temperature-sensitive paralytic mutant defines a primary synaptic calcium channel in Drosophila. J Neurosci. 2000;20:4885–4889. doi: 10.1523/JNEUROSCI.20-13-04885.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki F, Zou B, Xu X, Ordway RW. Active zone localization of presynaptic calcium channels encoded by the cacophony locus of Drosophila. J Neurosci. 2004;24:282–285. doi: 10.1523/JNEUROSCI.3553-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Bao H, Bonanno L, Zhang B, Serpe M. Drosophila Neto is essential for clustering glutamate receptors at the neuromuscular junction. Genes Dev. 2012;26:974–987. doi: 10.1101/gad.185165.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kner P, Chhun BB, Griffis ER, Winoto L, Gustafsson MG. Super-resolution video microscopy of live cells by structured illumination. Nat Methods. 2009;6:339–342. doi: 10.1038/nmeth.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll B, Drescher U. Src family kinases are involved in EphA receptor-mediated retinal axon guidance. J Neurosci. 2004;24:6248–6257. doi: 10.1523/JNEUROSCI.0985-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh TW, Verstreken P, Bellen HJ. Dap160/intersectin acts as a stabilizing scaffold required for synaptic development and vesicle endocytosis. Neuron. 2004;43:193–205. doi: 10.1016/j.neuron.2004.06.029. [DOI] [PubMed] [Google Scholar]
- Korkut C, Ataman B, Ramachandran P, Ashley J, Barria R, Gherbesi N, Budnik V. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell. 2009;139:393–404. doi: 10.1016/j.cell.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni SJ, Hall JC. Behavioral and cytogenetic analysis of the cacophony courtship song mutant and interacting genetic variants in Drosophila melanogaster. Genetics. 1987;115:461–475. doi: 10.1093/genetics/115.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurshan PT, Oztan A, Schwarz TL. Presynaptic alpha2delta-3 is required for synaptic morphogenesis independent of its Ca2+-channel functions. Nat Neurosci. 2009;12:1415–1423. doi: 10.1038/nn.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre S, Burlet P, Liu Q, Bertrandy S, Clermont O, Munnich A, Dreyfuss G, Melki J. Correlation between severity and SMN protein level in spinal muscular atrophy. Nat Genet. 1997;16:265–269. doi: 10.1038/ng0797-265. [DOI] [PubMed] [Google Scholar]
- Lin DM, Goodman CS. Ectopic and increased expression of Fasciclin II alters motoneuron growth cone guidance. Neuron. 1994;13:507–523. doi: 10.1016/0896-6273(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- Marder E. Neuromodulation of neuronal circuits: back to the future. Neuron. 2012;76:1–11. doi: 10.1016/j.neuron.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder E, Goaillard JM. Variability, compensation and homeostasis in neuron and network function. Nat Rev Neurosci. 2006;7:563–574. doi: 10.1038/nrn1949. [DOI] [PubMed] [Google Scholar]
- Marek KW, Davis GW. Transgenically encoded protein photoinactivation (FlAsH-FALI): acute inactivation of synaptotagmin I. Neuron. 2002;36:805–813. doi: 10.1016/s0896-6273(02)01068-1. [DOI] [PubMed] [Google Scholar]
- Marie B, Pym E, Bergquist S, Davis GW. Synaptic homeostasis is consolidated by the cell fate gene gooseberry, a Drosophila pax3/7 homolog. J Neurosci. 2010;30:8071–8082. doi: 10.1523/JNEUROSCI.5467-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marie B, Sweeney ST, Poskanzer KE, Roos J, Kelly RB, Davis GW. Dap160/intersectin scaffolds the periactive zone to achieve high-fidelity endocytosis and normal synaptic growth. Neuron. 2004;43:207–219. doi: 10.1016/j.neuron.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Marrus SB, Portman SL, Allen MJ, Moffat KG, DiAntonio A. Differential localization of glutamate receptor subunits at the Drosophila neuromuscular junction. J Neurosci. 2004;24:1406–1415. doi: 10.1523/JNEUROSCI.1575-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew D, Ataman B, Chen J, Zhang Y, Cumberledge S, Budnik V. Wingless signaling at synapses is through cleavage and nuclear import of receptor DFrizzled2. Science. 2005;310:1344–1347. doi: 10.1126/science.1117051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe BD, Marques G, Haghighi AP, Fetter RD, Crotty ML, Haerry TE, Goodman CS, O’Connor MB. The BMP homolog Gbb provides a retrograde signal that regulates synaptic growth at the Drosophila neuromuscular junction. Neuron. 2003;39:241–254. doi: 10.1016/s0896-6273(03)00426-4. [DOI] [PubMed] [Google Scholar]
- Menon KP, Andrews S, Murthy M, Gavis ER, Zinn K. The translational repressors Nanos and Pumilio have divergent effects on presynaptic terminal growth and postsynaptic glutamate receptor subunit composition. J Neurosci. 2009;29:5558–5572. doi: 10.1523/JNEUROSCI.0520-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miech C, Pauer HU, He X, Schwarz TL. Presynaptic local signaling by a canonical wingless pathway regulates development of the Drosophila neuromuscular junction. J Neurosci. 2008;28:10875–10884. doi: 10.1523/JNEUROSCI.0164-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M, Davis GW. Transsynaptic control of presynaptic Ca(2)(+) influx achieves homeostatic potentiation of neurotransmitter release. Curr Biol. 2012;22:1102–1108. doi: 10.1016/j.cub.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M, Liu KS, Sigrist SJ, Davis GW. RIM Controls Homeostatic Plasticity through Modulation of the Readily-Releasable Vesicle Pool. J Neurosci. 2012;32:16574–16585. doi: 10.1523/JNEUROSCI.0981-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M, Pym EC, Tong A, Davis GW. Rab3-GAP controls the progression of synaptic homeostasis at a late stage of vesicle release. Neuron. 2011;69:749–762. doi: 10.1016/j.neuron.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy VN, Schikorski T, Stevens CF, Zhu Y. Inactivity produces increases in neurotransmitter release and synapse size. Neuron. 2001;32:673–682. doi: 10.1016/s0896-6273(01)00500-1. [DOI] [PubMed] [Google Scholar]
- O’Brien RJ, Kamboj S, Ehlers MD, Rosen KR, Fischbach GD, Huganir RL. Activity-dependent modulation of synaptic AMPA receptor accumulation. Neuron. 1998;21:1067–1078. doi: 10.1016/s0896-6273(00)80624-8. [DOI] [PubMed] [Google Scholar]
- Ophoff RA, Terwindt GM, Vergouwe MN, van Eijk R, Oefner PJ, Hoffman SM, Lamerdin JE, Mohrenweiser HW, Bulman DE, Ferrari M, et al. Familial hemiplegic migraine and episodic ataxia type-2 are caused by mutations in the Ca2+ channel gene CACNL1A4. Cell. 1996;87:543–552. doi: 10.1016/s0092-8674(00)81373-2. [DOI] [PubMed] [Google Scholar]
- Osterwalder T, Yoon KS, White BH, Keshishian H. A conditional tissue-specific transgene expression system using inducible GAL4. Proc Natl Acad Sci U S A. 2001;98:12596–12601. doi: 10.1073/pnas.221303298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pack-Chung E, Kurshan PT, Dickman DK, Schwarz TL. A Drosophila kinesin required for synaptic bouton formation and synaptic vesicle transport. Nat Neurosci. 2007;10:980–989. doi: 10.1038/nn1936. [DOI] [PubMed] [Google Scholar]
- Paradis S, Sweeney ST, Davis GW. Homeostatic control of presynaptic release is triggered by postsynaptic membrane depolarization. Neuron. 2001;30:737–749. doi: 10.1016/s0896-6273(01)00326-9. [DOI] [PubMed] [Google Scholar]
- Penney J, Tsurudome K, Liao EH, Elazzouzi F, Livingstone M, Gonzalez M, Sonenberg N, Haghighi AP. TOR is required for the retrograde regulation of synaptic homeostasis at the Drosophila neuromuscular junction. Neuron. 2012;74:166–178. doi: 10.1016/j.neuron.2012.01.030. [DOI] [PubMed] [Google Scholar]
- Perez-Otano I, Ehlers MD. Homeostatic plasticity and NMDA receptor trafficking. Trends Neurosci. 2005;28:229–238. doi: 10.1016/j.tins.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Petersen SA, Fetter RD, Noordermeer JN, Goodman CS, DiAntonio A. Genetic analysis of glutamate receptors in Drosophila reveals a retrograde signal regulating presynaptic transmitter release. Neuron. 1997;19:1237–1248. doi: 10.1016/s0896-6273(00)80415-8. [DOI] [PubMed] [Google Scholar]
- Pietrobon D. CaV2.1 channelopathies. Pflugers Arch. 2010;460:375–393. doi: 10.1007/s00424-010-0802-8. [DOI] [PubMed] [Google Scholar]
- Pilgram GS, Potikanond S, van der Plas MC, Fradkin LG, Noordermeer JN. The RhoGAP crossveinless-c interacts with Dystrophin and is required for synaptic homeostasis at the Drosophila neuromuscular junction. J Neurosci. 2011;31:492–500. doi: 10.1523/JNEUROSCI.4732-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomp JJ, Van Kempen GT, De Baets MB, Graus YM, Kuks JB, Molenaar PC. Acetylcholine release in myasthenia gravis: regulation at single end-plate level. Ann Neurol. 1995;37:627–636. doi: 10.1002/ana.410370513. [DOI] [PubMed] [Google Scholar]
- Plomp JJ, van Kempen GT, Molenaar PC. Adaptation of quantal content to decreased postsynaptic sensitivity at single endplates in alpha-bungarotoxin-treated rats. J Physiol. 1992;458:487–499. doi: 10.1113/jphysiol.1992.sp019429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomp JJ, van Kempen GT, Molenaar PC. The upregulation of acetylcholine release at endplates of alpha-bungarotoxin-treated rats: its dependency on calcium. J Physiol. 1994;478(Pt 1):125–136. doi: 10.1113/jphysiol.1994.sp020236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poskanzer KE, Marek KW, Sweeney ST, Davis GW. Synaptotagmin I is necessary for compensatory synaptic vesicle endocytosis in vivo. Nature. 2003;426:559–563. doi: 10.1038/nature02184. [DOI] [PubMed] [Google Scholar]
- Pozo K, Goda Y. Unraveling mechanisms of homeostatic synaptic plasticity. Neuron. 2010;66:337–351. doi: 10.1016/j.neuron.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendra TK, Gonsalvez GB, Walker MP, Shpargel KB, Salz HK, Matera AG. A Drosophila melanogaster model of spinal muscular atrophy reveals a function for SMN in striated muscle. J Cell Biol. 2007;176:831–841. doi: 10.1083/jcb.200610053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando TA. The dystrophin-glycoprotein complex, cellular signaling, and the regulation of cell survival in the muscular dystrophies. Muscle Nerve. 2001;24:1575–1594. doi: 10.1002/mus.1192. [DOI] [PubMed] [Google Scholar]
- Roman G, Endo K, Zong L, Davis RL. P[Switch], a system for spatial and temporal control of gene expression in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2001;98:12602–12607. doi: 10.1073/pnas.221303998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross CA, Margolis RL, Reading SA, Pletnikov M, Coyle JT. Neurobiology of schizophrenia. Neuron. 2006;52:139–153. doi: 10.1016/j.neuron.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Sahin M, Greer PL, Lin MZ, Poucher H, Eberhart J, Schmidt S, Wright TM, Shamah SM, O’Connell S, Cowan CW, et al. Eph-dependent tyrosine phosphorylation of ephexin1 modulates growth cone collapse. Neuron. 2005;46:191–204. doi: 10.1016/j.neuron.2005.01.030. [DOI] [PubMed] [Google Scholar]
- Sakane A, Manabe S, Ishizaki H, Tanaka-Okamoto M, Kiyokage E, Toida K, Yoshida T, Miyoshi J, Kamiya H, Takai Y, et al. Rab3 GTPase-activating protein regulates synaptic transmission and plasticity through the inactivation of Rab3. Proc Natl Acad Sci U S A. 2006;103:10029–10034. doi: 10.1073/pnas.0600304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamah SM, Lin MZ, Goldberg JL, Estrach S, Sahin M, Hu L, Bazalakova M, Neve RL, Corfas G, Debant A, et al. EphA receptors regulate growth cone dynamics through the novel guanine nucleotide exchange factor ephexin. Cell. 2001;105:233–244. doi: 10.1016/s0092-8674(01)00314-2. [DOI] [PubMed] [Google Scholar]
- Shi L, Butt B, Ip FC, Dai Y, Jiang L, Yung WH, Greenberg ME, Fu AK, Ip NY. Ephexin1 is required for structural maturation and neurotransmission at the neuromuscular junction. Neuron. 2010a;65:204–216. doi: 10.1016/j.neuron.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Fu AK, Ip NY. Multiple roles of the Rho GEF ephexin1 in synapse remodeling. Commun Integr Biol. 2010b;3:622–624. doi: 10.4161/cib.3.6.13481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeath JB, Zhang Y, Holmgren R, Carroll SB, Doe CQ. Specification of neuroblast identity in the Drosophila embryonic central nervous system by gooseberry-distal. Nature. 1995;376:427–430. doi: 10.1038/376427a0. [DOI] [PubMed] [Google Scholar]
- Sleigh JN, Buckingham SD, Esmaeili B, Viswanathan M, Cuppen E, Westlund BM, Sattelle DB. A novel Caenorhabditis elegans allele, smn-1(cb131), mimicking a mild form of spinal muscular atrophy, provides a convenient drug screening platform highlighting new and pre-approved compounds. Hum Mol Genet. 2011;20:245–260. doi: 10.1093/hmg/ddq459. [DOI] [PubMed] [Google Scholar]
- Smith LA, Peixoto AA, Kramer EM, Villella A, Hall JC. Courtship and visual defects of cacophony mutants reveal functional complexity of a calcium-channel alpha1 subunit in Drosophila. Genetics. 1998;149:1407–1426. doi: 10.1093/genetics/149.3.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LA, Wang X, Peixoto AA, Neumann EK, Hall LM, Hall JC. A Drosophila calcium channel alpha1 subunit gene maps to a genetic locus associated with behavioral and visual defects. J Neurosci. 1996;16:7868–7879. doi: 10.1523/JNEUROSCI.16-24-07868.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2009;136:731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starcevic M, Dell’Angelica EC. Identification of snapin and three novel proteins (BLOS1, BLOS2, and BLOS3/reduced pigmentation) as subunits of biogenesis of lysosome-related organelles complex-1 (BLOC-1) J Biol Chem. 2004;279:28393–28401. doi: 10.1074/jbc.M402513200. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Südhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Ito HT, Cressy P, Kempf C, Woo JC, Schuman EM. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell. 2006;125:785–799. doi: 10.1016/j.cell.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Tedford HW, Zamponi GW. Direct G protein modulation of Cav2 calcium channels. Pharmacol Rev. 2006;58:837–862. doi: 10.1124/pr.58.4.11. [DOI] [PubMed] [Google Scholar]
- Thiagarajan TC, Lindskog M, Tsien RW. Adaptation to synaptic inactivity in hippocampal neurons. Neuron. 2005;47:725–737. doi: 10.1016/j.neuron.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Timmerman C, Sanyal S. Behavioral and electrophysiological outcomes of tissue-specific Smn knockdown in Drosophila melanogaster. Brain Res. 2012 doi: 10.1016/j.brainres.2012.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunoda S, Salkoff L. Genetic analysis of Drosophila neurons: Shal, Shaw, and Shab encode most embryonic potassium currents. J Neurosci. 1995;15:1741–1754. doi: 10.1523/JNEUROSCI.15-03-01741.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsurudome K, Tsang K, Liao EH, Ball R, Penney J, Yang JS, Elazzouzi F, He T, Chishti A, Lnenicka G, et al. The Drosophila miR-310 cluster negatively regulates synaptic strength at the neuromuscular junction. Neuron. 2010;68:879–893. doi: 10.1016/j.neuron.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano G. Homeostatic synaptic plasticity: local and global mechanisms for stabilizing neuronal function. Cold Spring Harb Perspect Biol. 2012;4:a005736. doi: 10.1101/cshperspect.a005736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- van der Plas MC, Pilgram GS, Plomp JJ, de Jong A, Fradkin LG, Noordermeer JN. Dystrophin is required for appropriate retrograde control of neurotransmitter release at the Drosophila neuromuscular junction. J Neurosci. 2006;26:333–344. doi: 10.1523/JNEUROSCI.4069-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstreken P, Kjaerulff O, Lloyd TE, Atkinson R, Zhou Y, Meinertzhagen IA, Bellen HJ. Endophilin mutations block clathrin-mediated endocytosis but not neurotransmitter release. Cell. 2002;109:101–112. doi: 10.1016/s0092-8674(02)00688-8. [DOI] [PubMed] [Google Scholar]
- Verstreken P, Koh TW, Schulze KL, Zhai RG, Hiesinger PR, Zhou Y, Mehta SQ, Cao Y, Roos J, Bellen HJ. Synaptojanin is recruited by endophilin to promote synaptic vesicle uncoating. Neuron. 2003;40:733–748. doi: 10.1016/s0896-6273(03)00644-5. [DOI] [PubMed] [Google Scholar]
- Weyhersmüller A, Hallermann S, Wagner N, Eilers J. Rapid active zone remodeling during synaptic plasticity. J Neurosci. 2011;31:6041–6052. doi: 10.1523/JNEUROSCI.6698-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildemann B, Bicker G. Developmental expression of nitric oxide/cyclic GMP synthesizing cells in the nervous system of Drosophila melanogaster. J Neurobiol. 1999a;38:1–15. doi: 10.1002/(sici)1097-4695(199901)38:1<1::aid-neu1>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Wildemann B, Bicker G. Nitric oxide and cyclic GMP induce vesicle release at Drosophila neuromuscular junction. J Neurobiol. 1999b;39:337–346. doi: 10.1002/(sici)1097-4695(19990605)39:3<337::aid-neu1>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Wu CF, Ganetzky B, Haugland FN, Liu AX. Potassium currents in Drosophila: different components affected by mutations of two genes. Science. 1983;220:1076–1078. doi: 10.1126/science.6302847. [DOI] [PubMed] [Google Scholar]
- Yoshihara M, Adolfsen B, Galle KT, Littleton JT. Retrograde signaling by Syt 4 induces presynaptic release and synapse-specific growth. Science. 2005;310:858–863. doi: 10.1126/science.1117541. [DOI] [PubMed] [Google Scholar]
- Zhang B, Koh YH, Beckstead RB, Budnik V, Ganetzky B, Bellen HJ. Synaptic vesicle size and number are regulated by a clathrin adaptor protein required for endocytosis. Neuron. 1998;21:1465–1475. doi: 10.1016/s0896-6273(00)80664-9. [DOI] [PubMed] [Google Scholar]
- Zhao C, Dreosti E, Lagnado L. Homeostatic synaptic plasticity through changes in presynaptic calcium influx. J Neurosci. 2011;31:7492–7496. doi: 10.1523/JNEUROSCI.6636-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Y, Pena LA. A novel synaptic transmission mediated by a PACAP-like neuropeptide in Drosophila. Neuron. 1995;14:527–536. doi: 10.1016/0896-6273(95)90309-7. [DOI] [PubMed] [Google Scholar]
- Zhuchenko O, Bailey J, Bonnen P, Ashizawa T, Stockton DW, Amos C, Dobyns WB, Subramony SH, Zoghbi HY, Lee CC. Autosomal dominant cerebellar ataxia (SCA6) associated with small polyglutamine expansions in the alpha 1A-voltage-dependent calcium channel. Nat Genet. 1997;15:62–69. doi: 10.1038/ng0197-62. [DOI] [PubMed] [Google Scholar]