Fig. 3.
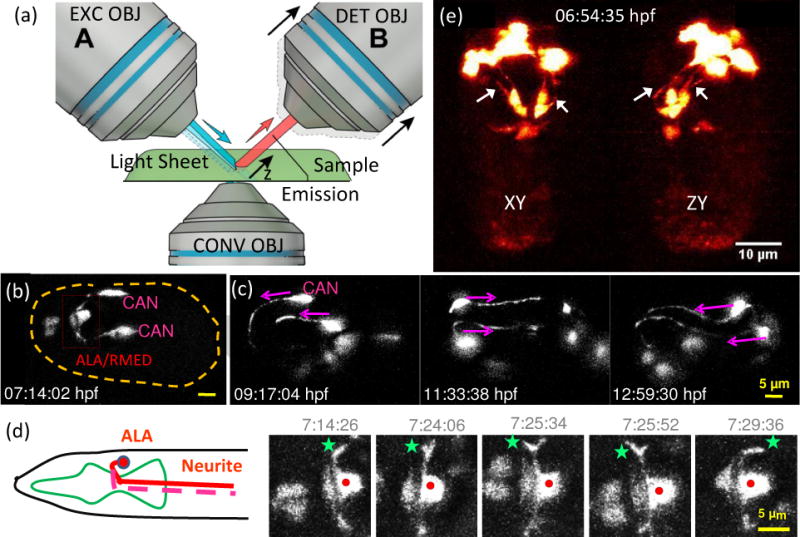
Light sheet fluorescence microscopy enables high-speed, long-term neurodevelopmental imaging during embryogenesis. (a) Inverted selective plane illumination microcopy (iSPIM) schematic. Two long working distance, water immersion objective lenses enable orthogonal SPIM excitation and detection. The excitation objective (EXC OBJ) introduces a light sheet at the sample, and the resulting fluorescence emission is collected by the detection objective (DET OBJ). High-speed volumetric imaging is achieved by sweeping the light sheet and focal plane through the sample, along the detection axis. The bottom objective provides an additional view of the sample and allows other optical modalities. iSPIM enables conventional mounting of specimens. (b–d) Visualization of neuron migration and neurite outgrowths in C.elegans embryos with iSPIM: (b) Maximum-intensity projections of ceh-10p:GFP, highlighting ALA/RMED and CAN neurons before twitching. Scale bar, 5 μm. (c) Time series of the CAN neurite outgrowth through the entire twitching period. (d) The cartoon shows the ALA neuron in the adult worm. Both neurites of ALA project towards the posterior end of the animal. The time-series images display a higher magnification view of the red box in (b). Red dot: ALA soma, green star: left neurite outgrowth. The images show the neuronal outgrowth of ALA through twitching and reveal when the bilateral neurites project towards the posterior end of the embryo. (e) Dual-view iSPIM (diSPIM) imaging highlighting GFP-tagged AIY neurons. Arrows indicate AIY neurites, clearly visible in both lateral projection (left image) and axial projection (right image). Panel c-d are adapted from Y. Wu, et al. [35] with permission from PNAS.
