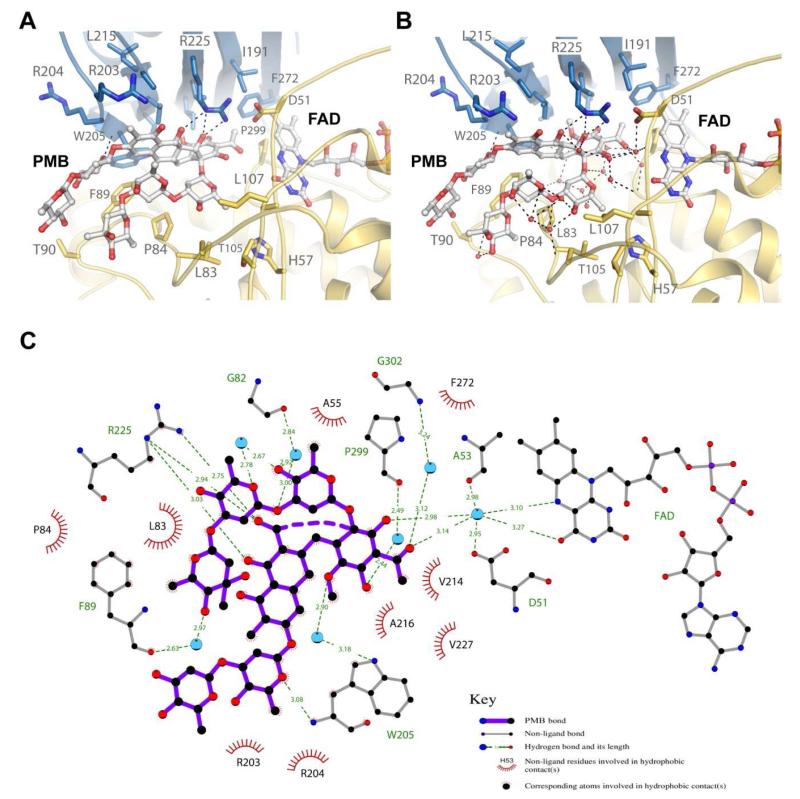
Figure 4. The premithramycin B binding site in MtmOIV.
A. Shown are the interactions (dashed lines) of MtmOIV with premithramycin B (PMB) bound along the active site with water molecules removed from structure. Without water molecules, there appear to be two primary interactions with W205 and R225, with van der Waals and hydrophobic interactions contributing a large part to the binding energy. B. Shown are the interactions of MtmOIV with PMB, including the water molcules that were observed in the crystal structure (1.85 Å resolution). Once solvation is included, a vast number of interactions (dashed lines, distance cutoff of 3.3 Å) are observed which are bridged by ordered water molecules, indicating that hydrogen bonding networks play a major role in substrate binding. C. Ligplot of PMB bound to MtmOIV indicating important interactions (dashed lines, distance cutoff of 3.3 Å). For clarity, atom names for PMB have been removed and only water molecules (cyan spheres) having two or more hydrogen bonding interactions are shown.