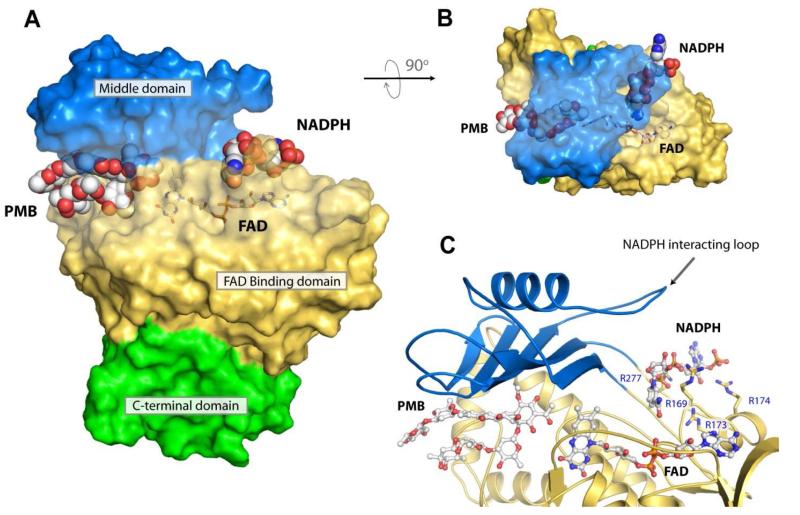
Figure 7. NADPH docked into the putative NADPH binding pocket in MtmOIV.
A. The MtmOIV structure showing the bound premithramycin B in the substrate binding pocket and the lowest energy docked model (−2.8 kcal/mol) of NADPH positioned within the putative NADPH binding pocket. B. Top-down view of the MtmOIV structure shown in panel A through the middle domain (blue). The binding pockets in panel A and B are shown as transparent surface and for clarity, FAD is shown in stick. C. Close-up view of the putative NADPH binding site shown in proximity to FAD and PMB (ball and stick) which was co-crystallized within the substrate binding pocket. Middle domain loop consisting of residues 233-239 has been postulated to participate in binding NADPH (ball and stick) and was found disordered in all known structures of MtmOIV except our low resolution NADPH co-crystal structure. Those residues that were identified from our low resolution crystal structure to interact with NADPH (R169, R173, R174, R277) are shown in stick representation.