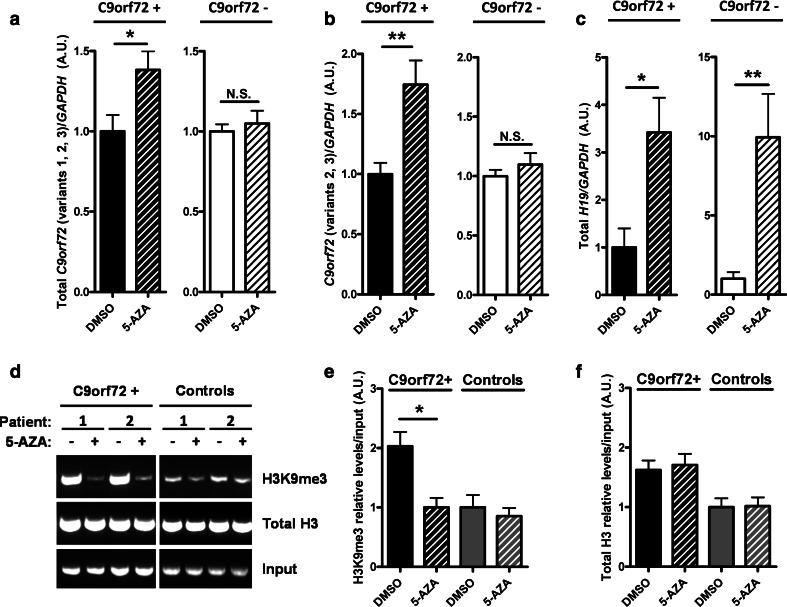
Fig. 3.
C9orf72 mRNA expression is increased and H3K9me3 binding is decreased in C9orf72+ fibroblasts upon 5-AZA treatment. a, b qRT-PCR of RNA obtained from human fibroblasts grown in DMSO or 5-AZA demethylating agent. Both assays targeting transcript variants 1, 2, 3 (a) and 2, 3 (b) show a significant increase in expression after 5-AZA treatment only in C9orf72 repeat expansion carriers. c qRT-PCR of H19, an imprinted gene, showing effectiveness of the 5-AZA treatment in C9orf72+ and C9orf72− fibroblasts. Statistical differences were assessed by unpaired Student t test. *p < 0.05, **p < 0.01. NS no significant difference. d Electrophoretic representation of chromatin immunoprecipitated DNA from a fibroblast subgroup using antibodies specific for total H3 or trimethylated histone H3K9. Fibroblasts were grown in DMSO or 5-AZA. Chromatin immunoprecipitation (ChIP) was performed in fibroblasts from C9orf72+ and C9orf72− participants. Following pull-down, bound DNA was purified and used for PCR amplification of the C9orf72 promoter region. Upon treatment with vehicle (DMSO), the binding to trimethylated histone H3K9 in C9orf72+ cells, as assessed by the level of amplified C9orf72 promoter region, was higher as compared to C9orf72−. Treatment with 5-AZA reduced this binding in C9orf72+ cases. The complete figure of all fibroblast lines is provided in the online resource. e, f Relative quantifications of all fibroblast lines were performed by measuring band intensity (complete gels in the online resource, Fig. 3) for each immunoprecipitated histone and presented as a ratio to the input. Graphs are normalized to total histone levels of disease control group (mean value set to 1). Statistical differences were calculated by one-way ANOVA with Tukey post hoc test. *p < 0.05. A clinical description of participants is available in Table 3 (online resource)