Abstract
Purpose
We evaluated and compared the effectiveness of an enuresis alarm, desmopressin medication, and their combination in the treatment of Saudi children with primary monosymptomatic nocturnal enuresis (PMNE).
Materials and Methods
A total of 136 children with PMNE were randomly assigned to receive an enuresis alarm alone (EA group, n=45), desmopressin alone (D group, n=46), or a combination of both (EA/D group, n=45). Patients were followed weekly during treatment and for 12 weeks after treatment withdrawal.
Results
During treatment, wetting frequencies were significantly reduced in all groups and remained significantly lower than pretreatment values until the end of follow-up. In the D and EA/D groups, an immediate reduction in wetting frequencies was observed, whereas a longer time was required to reach a significant reduction in the EA group. The full and partial response rates were 13.3% and 37.8% in the EA group, 26.1% and 43.5% in the D group, and 40.0% and 33.3% in the EA/D group. A significant difference was observed only between the EA and EA/D groups (p=0.025). Relapse rates were higher in the D group (66.6%) than in the EA (16.6%) and EA/D (33.3%) groups. A significant difference was observed between the D and EA groups only (p=0.019).
Conclusions
Desmopressin, an enuresis alarm, and combined therapy are effective in the treatment of Saudi children with PMNE. Desmopressin produced an immediate effect but relapses were common. The enuresis alarm provided gradual effects that persisted posttreatment. The combined therapy was superior to the alarm in achieving an immediate response; however, its effect was not better than that of the alarm long term.
Keywords: Combined modality therapy, Nocturnal enuresis, Treatment efficacy
INTRODUCTION
Bedwetting or nocturnal enuresis (NE) is the most common childhood urologic complaint and the most common issue reported at school examinations [1]. NE affects 10% to 15% of those aged 5 years, 5% of those aged 10 years, and 1% of those aged 15 years [2,3]. It can have a significant effect on the child or young person's behavior and emotional well-being. It may cause a child to be marginalized by family and friends and may instigate anger, punishments, and rejection in caregivers, culminating in a loss of the child's self-confidence [4,5]. Such psychoemotional effects have been found to improve after treatment of NE [6].
According to the diagnostic and statistical manual of mental disorders, 4th edition (DSM-IV) [7], NE is defined as bedwetting with a severity of at least twice per week in children over 5 years of age when not provoked by congenital or acquired defects of the central nervous system or by the direct physiological effect of a substance (such as a diuretic). It has been divided into primary NE (having never experienced a lengthy spell of dry nights) and secondary NE (having a history of being dry for at least 6 months) [5]. The occurrence of NE seems to be determined by 3 main factors that direct treatment implications: 1) nocturnal polyuria, which suggests a need for treatment with desmopressin; 2) nocturnal bladder reservoir function, which indicates a need for a bladder-training program coupled with anticholinergic treatment (e.g., oxybutynin); and 3) the arousal response to bladder fullness during sleep, which suggests that treatment with an enuresis alarm may be a good treatment option [8]. The clinical efficacy of these treatment strategies has been confirmed in previous studies. However, one should be cautious in extrapolating the findings of these studies to other communities given the possible differences in socioeconomic conditions that affect the clinical outcome. Because local Saudi data on the usefulness of these treatment strategies for NE are scanty, we conducted this study to determine and compare the clinical efficacy of an enuresis alarm, desmopressin, and the combination of both regimens in the treatment of Saudi children with primary monosymptomatic nocturnal enuresis (PMNE).
MATERIALS AND METHODS
1. Study design, patient selection, and assessment
This multicenter, prospective, randomized, comparative study was conducted between October 2009 and March 2013. Patients were consecutively enrolled from patients referred to the urology and pediatric clinics at Salman Bin Abdul-Aziz University Hospital, Al-Kharj; Royal Commission Hospital, Jubail; and King Khalid Hospitals, AL-Kharj and Tabouk, Kingdom of Saudi Arabia, for treatment of enuresis. The size of the research sample was determined by using the GraphPad Prism sample size calculator (GraphPad Software, La Jolla, CA, USA). On the basis of previous studies [9-15] of the trial parameters (reduction in wetting frequencies, response to treatment, relapse rates, etc.), a clinically important difference of 30% in the proportion of these parameters was considered acceptable. We considered a dropout rate of 10%. To obtain a significance level of 0.05 and a power of 80%, a sample size of 40 patients per group was needed. All patients underwent thorough history-taking including a lower urinary tract symptoms (LUTS) questionnaire, physical examination, urinalysis, midstream urine culture, and measurement of serum creatinine and serum electrolytes. The DSM-IV criteria [7] were used to define children with NE. The severity of NE was categorized as mild (2-3 times/wk), moderate (4-6 times/wk), and severe (7 times/wk).
2. Inclusion and exclusion criteria
Patients 6 to 14 years of age, having wetting episodes at least twice per week over 3 consecutive months, and whose parents agreed to allocation of treatment were eligible for enrollment. Patients with identifiable LUTS other than NE and those with other physical disorders, such as diabetes; with abnormal urine analysis results; with renal, urological, cardiovascular, or neurological diseases; or previously treated with an enuresis alarm or desmopressin were excluded from study.
3. Study procedure and flow of patients through the study
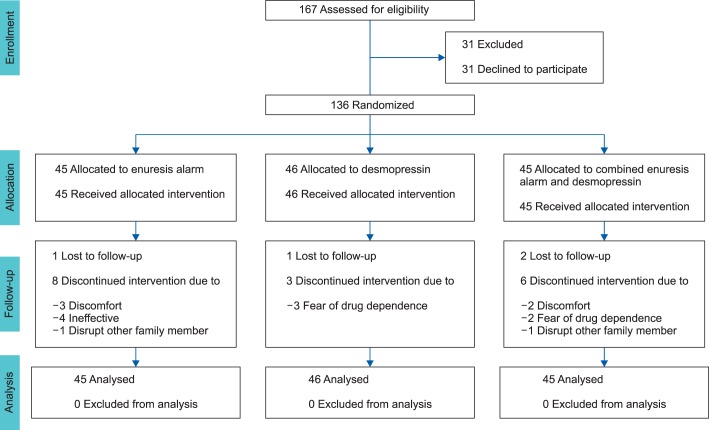
Out of 167 patients who met the inclusion criteria, 31 chose not to participate. The remaining 136 patients were randomly divided into three groups through a computer randomization program and received treatment for 12 weeks: group I, the enuresis alarm group (EA group, n=45); group II, the desmopressin group (D group, n=46); and group III, combined enuresis alarm and desmopressin group (EA/D group, n=45) (Fig. 1). To keep the groups closely balanced, we used randomization in blocks of three and each center had its own list. In patients using the alarm device, the children were instructed to test the alarm before sleep, with the sound (or vibration) in mind, and the families were instructed to awake the child immediately after onset of the alarm and to send him to the bathroom to finish voiding. In patients assigned to desmopressin, an oral lyophilisate pharmaceutical form of desmopressin (melt formulation) was used. The patients were advised to place the tablet under the tongue and to allow it to dissolve completely without water. They started on 120 µg 1 hour before bedtime. If no considerable response was achieved after 2 weeks (>1 wet night/wk), the doses were increased to 240 µg. All patients were advised to urinate just before going to bed and to restrict their fluid intake (not to drink more than sufficient to satisfy thirst) from 1 hour before bedtime. They were also instructed to avoid drinking liquids with a diuretic effect at night, to keep regular sleep hours, and to keep a record of night wetting episodes. In addition, the children used a star chart for recording the number of dry and wet nights and compliance with treatment.
FIG. 1.
The flow diagram.
The protocol and all study procedures were conducted in conformity with the ethical guidelines of the Declaration of Helsinki of 1975. The study protocol was approved by the ethics committee of the participating hospitals, and parents of all patients enrolled in this study provided written informed consent.
4. Follow-up
Follow-up visits were scheduled on a weekly basis during the treatment period and 3 months after treatment discontinuation. At each follow-up visit, blood pressure, response to and compliance with treatment, and adverse effects were recorded. Serum sodium was measured at the first follow-up visit and at the end of treatment.
5. Outcome measurements
Treatment efficacy was determined by comparison of wet nights in the pretreatment period with the numbers during the last week of treatment. Treatment outcomes were categorized as either success or failure on the basis of the 1-week nocturnal records at the end of treatment. Successful outcomes included the following responses, as defined by the Standardization Committee of the International Children's Continence Society (ICCS) [16]: full response, no wet nights; response, ≥90% reduction in the number of wet nights; partial response, 50% to 89% reduction in the number of wet nights. Treatment failure was defined as nonresponse (<50% reduction in the number of wet nights). A relapse was defined as more than one wetting episode per month after full response.
6. Statistical analysis
Among those assigned to therapy, treatment efficacy analyses were performed by using the intention-to-treat population, defined as all enrolled patients who received at least one dose of any study medication. Statistical analyses were performed by using paired or independent t-tests when appropriate for continuous variables and chi-square tests for categorical variables. The treatment outcomes (response, noncompliance, and relapse rates) were compared by using chi-square tests. The differences in the mean number of wetting episodes per week before treatment, at the end of treatment, and at the end of follow-up were assessed by using one-way analysis of variance (ANOVA). Within-group comparisons over these 3 time points were performed by paired t-test. Statistical analysis was done by using SAS ver. 16 (SAS Institute Inc., Cary, NC, USA), MedCalc ver. 12 (MedCalc Software, Ostend, Belgium), and GraphPad Prism.
RESULTS
As shown in Fig. 1, a total of 136 patients were enrolled in this study and 115 patients completed the treatment. The 21 patients who did not complete the study included 9 patients from group I, 4 patients from group II, and 8 patients from group III. Group I consisted of 32 boys and 13 girls (mean age, 8.21±2.05 years), group II consisted of 30 boys and 16 girls (mean age, 7.97±2.10 years), and group III consisted of 33 boys and 12 girls (mean age, 8.01±1.96 years). The mean number of wet nights/wk was 4.56±1.71 for group I, 5.04±1.58 for group II, and 4.73±1.66 for group III. Urine analysis and serum electrolytes were normal in all patients. There were no statistically significant differences between the 3 groups in terms of age, sex, mean wetting frequencies, or severity of NE (p>0.05). The baseline characteristics of the studied patients are summarized in Table 1.
TABLE 1.
Baseline characteristics of the studied patients
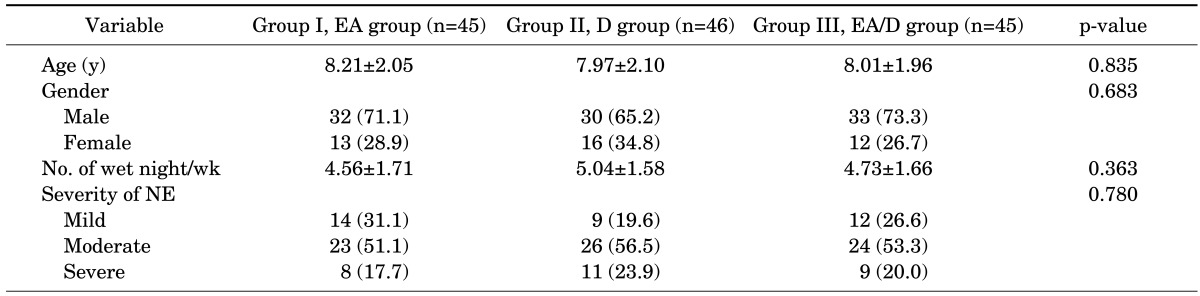
Values are presented as mean±standard deviation or number (%).
EA, enuresis alarm alone; D, desmopressin alone; EA/D, combination of enuresis alarm and desmopressin; NE, nocturnal enuresis.
At the end of treatment, wetting frequencies were significantly reduced in all groups, by 54.8%, 65.1%, and 78.9% in the groups EA, D, and EA/D, respectively (Table 2). Rebound occurred in all groups after discontinuation of treatment; however, the reduction in wetting frequencies remained significant until the end of follow-up (Fig. 2).
TABLE 2.
Mean number and percentage reduction of wet nights at the end of treatment and end of follow-up
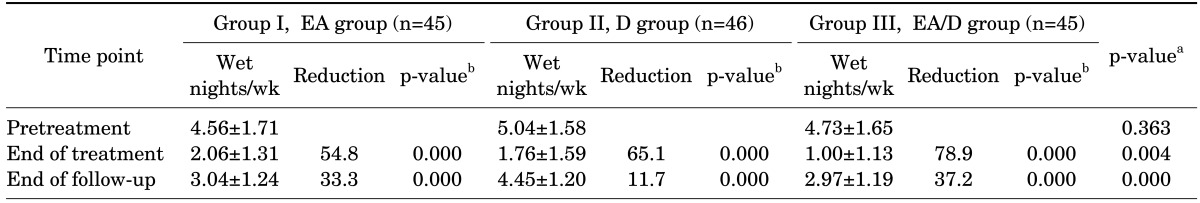
Values are presented as mean±standard deviation or percentage. Wetting frequencies at different time points were compared with pretreatment values within group by using paired t-tests. Wetting frequencies at different time points were compared between groups by using analysis of variance (ANOVA).
EA, enuresis alarm alone; D, desmopressin alone; EA/D, combination of enuresis alarm and desmopressin.
a:ANOVA test. b:Paired t-test.
FIG. 2.
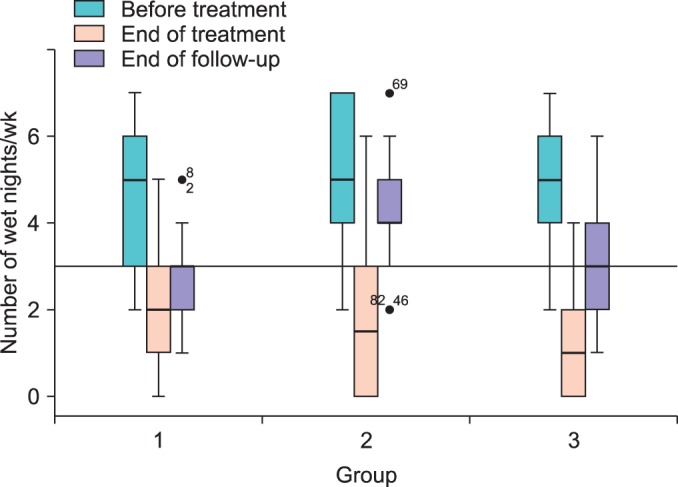
Box-and-whisker plot. Wetting frequencies, within-group, at different time points were compared with pretreatment values by using paired t-tests. A significant reduction in wet nights was observed between baseline, end of treatment, and end of follow-up in the 3 groups; p<0.0001 in all groups except between end of treatment and end of follow-up in group 2 (p=0.047).
The wetting frequencies were analyzed week by week during treatment and follow-up periods (Fig. 3). Groups D and EA/D patients showed immediate improvement and registered statistically significant reductions in wetting frequencies from week 1. Group EA patients showed gradual improvement and statistically significant reduction was observed only from week 4. At the end of treatment, the wetting frequencies were significantly higher in the group EA/D than in the groups EA and D (p<0.0001 and p=0.018, respectively). After discontinuation of treatment, the group D showed an immediate rebound of wetting and frequencies became higher than in the groups EA and EA/D until the end of follow-up. In the groups EA/D and EA, significant rebound was observed at follow-up weeks 3 and 10, respectively; there was no significant difference in wetting frequencies between the groups EA and EA/D from follow-up week 1 until the end of follow-up.
FIG. 3.
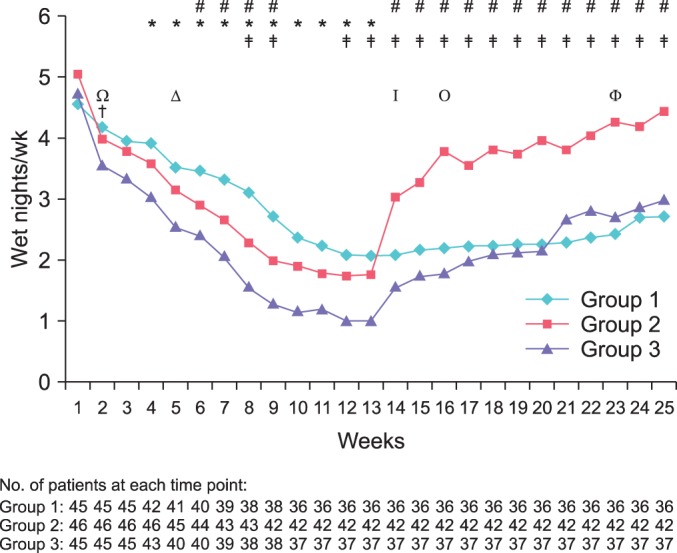
Wetting frequencies were analyzed week by week in the 3 groups by using paired and independent t-tests. Baseline (week 1); treatment period (weeks 2-13); follow-up period (weeks 14-25). (Δ), time of significant reduction of wet nights in group 1 (p=0.0001); (Ω), time of significant reduction in wet nights in group 2 (p=0.0001); (†), time of significant reduction of wet nights in group 3 (p<0.0001); (Φ), time of significant rebound in group 1 (p=0.0074); (I), time of significant rebound in group 2 (p=0.0009); (O), time of significant rebound in group 3 (p=0.0047); (*), p<0.05, intergroup comparison of group 1 vs. 2; (‡), p<0.05, intergroup comparison of group 1 vs. 3; (#), p <0.05, intergroup comparison of group 2 vs. 3.
Counting defaulters due to noncompliance issues as a form of failure (intent-to-treat analysis), the full response and partial response rates were 13.3% and 37.8% in the group EA, 26.1% and 43.5% in the group D, and 40.0% and 33.3% in the group EA/D, respectively. ANOVA showed that the full response to treatment was significantly higher in the group EA/D than in the group EA (p=0.025), whereas other group pairs were not significantly different. The relapse rates were higher in the group D (66.6%) than in the group EA (16.6%) or the group EA/D (33.3%). A significant difference was observed only between the groups EA and D (p=0.019). Table 3 shows the treatment outcomes in the 3 groups. Noncompliance rates were higher in the groups EA (20.0%) and EA/D (17.8%) than in the group D (8.7%), but the difference was not statistically significant (p=0.230, Fisher exact test). No adverse events were seen during the study period.
TABLE 3.
Treatment outcomes in the three groups
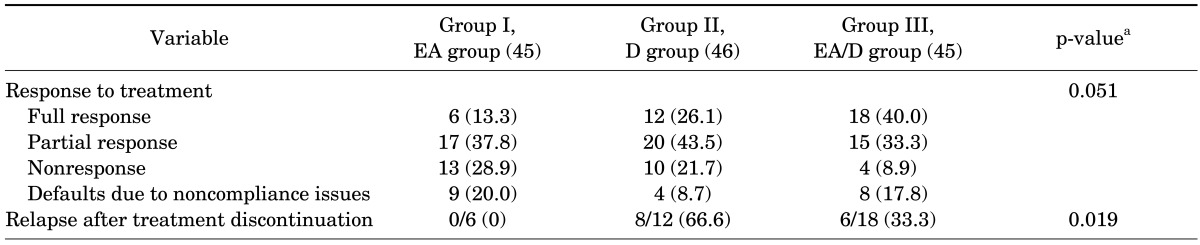
Values are presented as number (%). Chi-square test was used to calculate the significance of differences between the proportions. A statistically significant difference was observed between groups in relapse rates. Intergroup comparisons of treatment outcomes by analysis of variance (post hoc test): the response to treatment was significantly better in group III than in group I (p=0.025) and the relapse rate was significantly higher in group II than in group I (p=0.019); otherwise, the association was considered to be statistically nonsignificant.
EA, enuresis alarm alone; D, desmopressin alone; EA/D, combination of enuresis alarm and desmopressin.
a:Chi-square test.
DISCUSSION
This study was the first to evaluate and compare the efficacy of an enuresis alarm, desmopressin treatment, or a combination of both regimens for the treatment of enuretic Saudi children. Our results agreed with previous global patterns demonstrating that an enuresis alarm, desmopressin, and a combination of both are effective treatment modalities for Saudi children with PMNE. The average wetting frequencies of participants in the 3 groups were significantly reduced during treatment and 3 months after treatment when compared with pretreatment values. The percentage of patients who responded to treatment was 51.1%, 69.6%, and 73.3% in the groups EA, D, and EA/D, respectively, and response was sustained after treatment withdrawal in 83.4%, 33.4%, and 66.7.0% of full responders in the 3 groups, respectively.
Although several modalities (behavioral and pharmacological) are available for the treatment of primary NE, none has proven complete success, most likely as a result of incomplete understanding of the pathophysiology of NE [17]. Alarm therapy is an effective treatment strategy for primary NE owing to its efficacy, low relapse rates, and absence of side effects [18]. It is presumed to cure primary NE as a result of conditioning effects on arousal [19] or by increasing nocturnal bladder reservoir function [20]. The most important disadvantage of alarm treatment is the late onset of its impact; it starts to result in effects in a couple of weeks [14,21]. This might decrease the motivation of the child and family. In our study, the alarm device exerted a significant effect after 4 weeks. Also, the rates of noncompliance were higher in the groups treated with an alarm. The precise cause of this higher rate is unknown. However, some factors could have an effect on the response to alarm treatment, particularly those of a psychological nature, such as marital status conflict, lack of motivation, and parental penalty [21]. The physiological aspects related to a poor response to alarm treatment embody the problem of awakening with the sound of the device [15].
Another option in the treatment of primary NE is desmopressin medical therapy. Desmopressin was originally introduced for the treatment of central diabetes insipidus. Desmopressin has been available as intranasal spray and tablet formulations for the treatment of primary NE for many years; the convenient, sublingual oral lyophilisate (melt) formulation is a more recent development [22]. Desmopressin oral melt is the preferable formulation over tablet and requires no water intake and is associated with high compliance among children aged 5 to 11 years [23]. Overall, desmopressin is a safe drug with few side effects and low risks even when used for many years [24]. The only serious potential side effect that has been reported in children with NE who were treated with desmopressin is water intoxication with hyponatremia and convulsions. The chance of this complication is higher when the nasal spray is used [25]. Consequently, the enuresis indication has been removed for the nasal spray in several countries. Regardless of the formulation used, there are recognizable and preventable risk factors for hyponatremia. An unsuitably high fluid intake before desmopressin administration could be a key risk issue [26]. In our study, the oral melt tablet was used 1 hour before bedtime and evening fluids were allowed as desired with no side effects detected.
The efficacy of an alarm device and desmopressin in the treatment of primary NE was evaluated and compared in several previous studies [9,27]. Similar to these studies, we found that desmopressin elucidated a more rapid onset of action than the alarm, but desmopressin was associated with a significantly higher relapse rate.
The combined therapy was significantly better than alarm therapy, but not desmopressin therapy, in achieving an immediate short-term response; however, its effect was no better than the alarm in the long term. Three months after treatment discontinuation, there was no significant difference between the 2 groups concerning relapse rates. The rapid effect of desmopressin can increase patients' confidence and improve compliance. However, in the present study, no statistically significant difference was noted between the 3 groups in noncompliance rates. Our study results are in line with most previous studies' data and results. A British study [10] investigated 71 enuretic children and compared the use of combined desmopressin and alarm with alarm monotherapy and found that 76% of children receiving combined therapy became dry compared with 46% of those using the alarm alone, with similar relapse rates, 15% and 19%, respectively. In a placebo-controlled study, Leebeek-Groenewegen et al. [11] compared the efficacy of combined enuresis alarm and desmopressin with alarm monotherapy in the treatment of 93 enuretic children and found a significant reduction in wetting frequencies with combined therapy compared with alarm monotherapy. However, no significant difference was seen between the 2 groups in the relapse rates after treatment discontinuation. Sukhai et al. [12] reported their crossover trial of combined desmopressin and alarm versus placebo and alarm and showed a significant reduction in wet nights with combined therapy during the 2 weeks of observation compared with placebo and alarm. However, the 2-week treatment period was inadequate because alarm therapy takes as long as 5 to 8 weeks before success can be determined.
In the randomized comparative study of Fai-Ngo Ng et al. [13], 105 Chinese enuretic children received an enuresis alarm, desmopressin, or combined enuresis alarm and desmopressin therapy for 12 weeks and were followed for another 12 weeks. Patients with ≤1 wet night/wk in the last 4 weeks were defined as full responders, patients with >1 wet night/wk but a >50% reduction in the wetting frequency were defined as partial responders, and patients with a <50% reduction in wetting frequency were defined as nonresponders. Those authors determined that the total, full, and partial response rates were 42.9%, 22.9%, and 20% for alarm therapy; 52.5%, 24%, and 10.5% for desmopressin therapy; and 78.1%, 62.5%, and 15.6% for combined therapy, respectively. They attributed the lower effects especially with alarm and desmopressin monotherapy to the overcrowded living community of the Chinese population, which affects the motivation and compliance of families and children. Also, the possibility of high bladder dysfunction in Chinese children with primary NE may be a contributing factor [28]. They assessed the relapse rate as the percentage of total responders (complete plus partial) in each group when treatment was stopped and found no significant differences between the three groups regarding relapse rates. In our study, the relapse rates were significantly higher in the group D than in the group EA; all EA patients who fully responded to treatment remained dry until the end of follow-up. One study [29] reported that relapse rates with desmopressin in adults depend on the initial tailored dose; however, this needs to be confirmed with further studies on children. Moreover, the calculated results are greatly affected by the methods used for outcome measurement. This point may also explain the heterogeneity in the proportion of full and partial responders for each treatment modality in most of the studies. In the present study, the proportion of full to partial response rates seemed to be lower than in the previous report because we used the ICCS definitions to evaluate the response to treatment and only patients with no wet nights were considered full responders.
One limitation of the present study was the lack of an untreated control group, because the possibility of spontaneous resolution could not be excluded. In our departments we are not permitted, for ethical purposes, to exclude or to delay treatment of patients, especially if the treatment modalities used have been shown to be effective in previous studies. However, the main focus of the present study was to compare the different treatment strategies for PMNE and to evaluate whether the combination of enuresis alarm and desmopressin has a beneficial effect over either treatment alone. Another limitation was the short follow-up period, because 3 months of follow-up is insufficient for valid recommendation. Therefore, these results should be clinically applied with caution. Controlled studies with larger study populations and longer follow-up should be conducted to facilitate the standardization of the appropriate method for treatment of PMNE and to determine if the results in this study are maintained over time.
CONCLUSIONS
Desmopressin, enuresis alarm, and combined therapy are effective in the treatment of Saudi children with PMNE. Desmopressin produced an immediate effect but relapses were common. The enuresis alarm resulted in gradual effects that persisted posttreatment. Combined therapy showed better immediate response rates than did the alarm but no significant difference in relapse rates. We suggest that adding desmopressin to therapy with an enuresis alarm is of benefit only in achieving a rapid response if children or families seek rapid improvement.
ACKNOWLEDGMENTS
We gratefully acknowledge Dr. Sanaa Kamal, research study coordinator, for her assistance in statistics and typing of the manuscript. We also thank Elizabeth Bautista, urology nurse, for patient enrollment, specimen collection and reporting data.
Footnotes
The authors have nothing to disclose.
References
- 1.Yamamoto LG, Inaba AS, Okamoto JK, Patrinos ME, Yamashiroya VK, editors. Case based pediatrics for medical students and residents [monograph on the Internet] Honolulu: Department of Pediatrics, University of Hawaii John A Burns School of Medicine; c2004. [cited 2010 May 28]. Enuresis; pp. 5–28. Available from: http://www.hawaii.edu/medicine/pediatrics/pedtext/ [Google Scholar]
- 2.Butler RJ, Golding J, Northstone K ALSPAC Study Team. Nocturnal enuresis at 7.5 years old: prevalence and analysis of clinical signs. BJU Int. 2005;96:404–410. doi: 10.1111/j.1464-410X.2005.05640.x. [DOI] [PubMed] [Google Scholar]
- 3.Ozden C, Ozdal OL, Altinova S, Oguzulgen I, Urgancioglu G, Memis A. Prevalence and associated factors of enuresis in Turkish children. Int Braz J Urol. 2007;33:216–222. doi: 10.1590/s1677-55382007000200013. [DOI] [PubMed] [Google Scholar]
- 4.Butler RJ, Heron J. The prevalence of infrequent bedwetting and nocturnal enuresis in childhood: A large British cohort. Scand J Urol Nephrol. 2008;42:257–264. doi: 10.1080/00365590701748054. [DOI] [PubMed] [Google Scholar]
- 5.Butler RJ. Impact of nocturnal enuresis on children and young people. Scand J Urol Nephrol. 2001;35:169–176. [PubMed] [Google Scholar]
- 6.Longstaffe S, Moffatt ME, Whalen JC. Behavioral and self-concept changes after six months of enuresis treatment: a randomized, controlled trial. Pediatrics. 2000;105(4 Pt 2):935–940. [PubMed] [Google Scholar]
- 7.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV. Washington, DC: American Psychiatry Press; 1994. [Google Scholar]
- 8.Hjalmas K, Arnold T, Bower W, Caione P, Chiozza LM, von Gontard A, et al. Nocturnal enuresis: an international evidence based management strategy. J Urol. 2004;171(6 Pt 2):2545–2561. doi: 10.1097/01.ju.0000111504.85822.b2. [DOI] [PubMed] [Google Scholar]
- 9.Wille S. Comparison of desmopressin and enuresis alarm for nocturnal enuresis. Arch Dis Child. 1986;61:30–33. doi: 10.1136/adc.61.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradbury MG, Meadow SR. Combined treatment with enuresis alarm and desmopressin for nocturnal enuresis. Acta Paediatr. 1995;84:1014–1018. doi: 10.1111/j.1651-2227.1995.tb13818.x. [DOI] [PubMed] [Google Scholar]
- 11.Leebeek-Groenewegen A, Blom J, Sukhai R, Van Der Heijden B. Efficacy of desmopressin combined with alarm therapy for monosymptomatic nocturnal enuresis. J Urol. 2001;166:2456–2458. [PubMed] [Google Scholar]
- 12.Sukhai RN, Mol J, Harris AS. Combined therapy of enuresis alarm and desmopressin in the treatment of nocturnal enuresis. Eur J Pediatr. 1989;148:465–467. doi: 10.1007/BF00595916. [DOI] [PubMed] [Google Scholar]
- 13.Fai-Ngo Ng C, Wong SN Hong Kong Childhood Enuresis Study Group. Comparing alarms, desmopressin, and combined treatment in Chinese enuretic children. Pediatr Nephrol. 2005;20:163–169. doi: 10.1007/s00467-004-1708-5. [DOI] [PubMed] [Google Scholar]
- 14.Van Leerdam FJ, Blankespoor MN, Van Der Heijden AJ, Hirasing RA. Alarm treatment is successful in children with day- and night-time wetting. Scand J Urol Nephrol. 2004;38:211–215. doi: 10.1080/00365590410025460. [DOI] [PubMed] [Google Scholar]
- 15.Butler RJ, Robinson JC. Alarm treatment for childhood nocturnal enuresis: an investigation of within-treatment variables. Scand J Urol Nephrol. 2002;36:268–272. doi: 10.1080/003655902320248227. [DOI] [PubMed] [Google Scholar]
- 16.Nevéus T, von Gontard A, Hoebeke P, Hjalmas K, Bauer S, Bower W, et al. The standardization of terminology of lower urinary tract function in children and adolescents: report from the Standardisation Committee of the International Children's Continence Society. J Urol. 2006;176:314–324. doi: 10.1016/S0022-5347(06)00305-3. [DOI] [PubMed] [Google Scholar]
- 17.Husmann DA. Enuresis. Urology. 1996;48:184–193. doi: 10.1016/S0090-4295(96)00153-7. [DOI] [PubMed] [Google Scholar]
- 18.Lyon C, Schnall J. What is the best treatment for nocturnal enuresis in children? J Fam Pract. 2005;54:905–906. 909. [PubMed] [Google Scholar]
- 19.Butler RJ, Holland P, Gasson S, Norfolk S, Houghton L, Penney M. Exploring potential mechanisms in alarm treatment for primary nocturnal enuresis. Scand J Urol Nephrol. 2007;41:407–413. doi: 10.1080/00365590701571506. [DOI] [PubMed] [Google Scholar]
- 20.Oredsson AF, Jorgensen TM. Changes in nocturnal bladder capacity during treatment with the bell and pad for monosymptomatic nocturnal enuresis. J Urol. 1998;160:166–169. [PubMed] [Google Scholar]
- 21.Butler RJ, Gasson SL. Enuresis alarm treatment. Scand J Urol Nephrol. 2005;39:349–357. doi: 10.1080/00365590500220321. [DOI] [PubMed] [Google Scholar]
- 22.Adersson KE, Appell R, Cardozo L, Chapple C, Drutz H, Fourcroy J, et al. Pharmacological treatment of urinary incontinence. In: Abrams P, Cardozo L, Wein A, Khoury S, editors. Incontinence; 3rd International Consultation on Incontinence; 2004 June 26-29; Monaco. Plymouth: Plymbridge Distributors; 2005. pp. 809–854. [Google Scholar]
- 23.Lottmann H, Froeling F, Alloussi S, El-Radhi AS, Rittig S, Riis A, et al. A randomised comparison of oral desmopressin lyophilisate (MELT) and tablet formulations in children and adolescents with primary nocturnal enuresis. Int J Clin Pract. 2007;61:1454–1460. doi: 10.1111/j.1742-1241.2007.01493.x. [DOI] [PubMed] [Google Scholar]
- 24.Wolfish NM, Barkin J, Gorodzinsky F, Schwarz R. The Canadian Enuresis Study and Evaluation: short- and long-term safety and efficacy of an oral desmopressin preparation. Scand J Urol Nephrol. 2003;37:22–27. doi: 10.1080/00365590310008631. [DOI] [PubMed] [Google Scholar]
- 25.Robson WL, Leung AK, Norgaard JP. The comparative safety of oral versus intranasal desmopressin for the treatment of children with nocturnal enuresis. J Urol. 2007;178:24–30. doi: 10.1016/j.juro.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 26.Robson WL, Norgaard JP, Leung AK. Hyponatremia in patients with nocturnal enuresis treated with DDAVP. Eur J Pediatr. 1996;155:959–962. doi: 10.1007/BF02282887. [DOI] [PubMed] [Google Scholar]
- 27.Monda JM, Husmann DA. Primary nocturnal enuresis: a comparison among observation, imipramine, desmopressin acetate and bed-wetting alarm systems. J Urol. 1995;154(2 Pt 2):745–748. [PubMed] [Google Scholar]
- 28.Yeung CK, Sit FK, To LK, Chiu HN, Sihoe JD, Lee E, et al. Reduction in nocturnal functional bladder capacity is a common factor in the pathogenesis of refractory nocturnal enuresis. BJU Int. 2002;90:302–307. doi: 10.1046/j.1464-410x.2002.02884.x. [DOI] [PubMed] [Google Scholar]
- 29.Burgu B, Gokce MI, Gucuk A, Soygur T. Prospective evaluation of factors affecting the response and relapse rates to desmopressin therapy in male monosymptomatic enuretic adults. Urology. 2009;74:915–919. doi: 10.1016/j.urology.2009.05.033. [DOI] [PubMed] [Google Scholar]