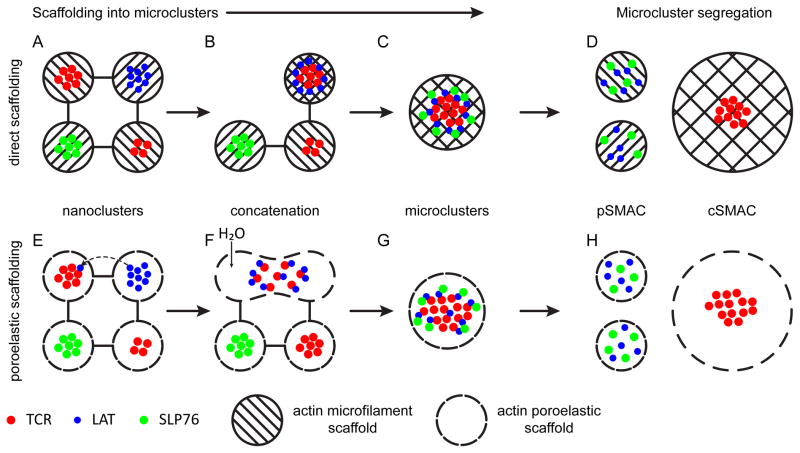
Fig. 1. Models of T-cell receptor scaffolding.
Two models of signaling microcluster scaffolding. (A–D) A direct, microfilament interaction-based model of signalosome scaffolding. (E–H) A poroelastic model of signalosome scaffolding. (A) TCRs and signaling effectors like LAT interact with the actin cytoskeleton, organizing into nanoclusters. (B) Upon triggering, actin cytoskeletal rearrangements allow protein islands of TCRs and LAT to concatenate. (C) Further concatenation and cytoskeletal remodeling leads to the aggregation of microclusters and the recruitment of other signaling effectors, such as SLP-76. (D) Association with signaling effectors is dependent on the actin cytoskeleton for scaffolding. As a result, as microclusters move into the cSMAC, signaling effectors are lost from TCR microclusters. In the pSMAC, the effectors can be rescaffolded with F-actin. (E) The actin cytoskeleton corrals TCRs into cytosolic pores. Confinement to a pore is not absolute, and diffusion into nearby pores can occur (dotted line). The rate at which molecules move into neighboring pores would be inversely proportional to the strength of the homotypic interactions holding molecules in nanoclusters and the hydrodynamic radius of the molecule. (F) As signaling is initiated, water influx, possibly induced by Na-H antiporter activity, changes the local hydrostatic pressure, causing pore deformation and swelling. This increases the mean pore size, allowing nanoclusters of TCRs and LAT to merge in growing pores. (G) Continued hydrostatic pressure facilitates the merging of TCR nanoclusters into microclusters and the incorporation of more signaling effector molecules, such as SLP-76 to sustain signaling. (H) In the low F-actin density interior of the synapse, effectors that require actin scaffolding to remain associated with TCRs release and are separated from TCRs by anterograde fluid flow. The molecules are free to diffuse back into smaller peripheral pores, terminating TCR signaling. Meanwhile, TCRs, which have become independent of the actin cytoskeleton for association in microclusters, remain trapped in the cSMAC. The TCRs cannot diffuse into the periphery of the synapse due to the fine pore size of the pSMAC.