Figure 1.
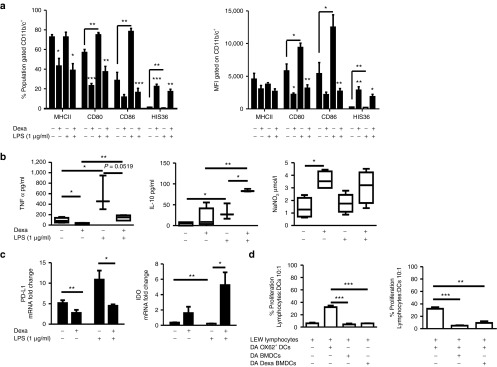
Phenotypic and functional characterization of immunomodulatory ex vivo–generated BMDCs and Dexa BMDCs. BMDCs were differentiated with GMCSF and IL-4 (5 ng/ml respectively) for 10 days in the absence or presence of Dexa (10−6 mol/l added to the culture on day 4). (a) Mean percentage and MFI of MHCII, CD80, CD86, and HIS36 expression (examined to monitor the presence of contaminating macrophage but known to be increased with glucocorticoid treatment within the CD11b/c+ population),50 BMDCs cultures were stimulated with LPS (1 µg/ml, 24 hours) (mean ± SEM n = 3 experiments *P ≤ 0.05 two-tailed Student's t-test). (b) Supernatants of BMDCs and Dexa BMDC were analyzed by ELISA for production of TNF-α, IL-10 and by Griess assay to measure nitrite (NO2−) production, before and after LPS stimulation (mean ± SEM *P ≤ 0.05 and **P ≤ 0.01 two-tailed Student's t-test n = 3–6). (c) mRNA expression (normalized to β-actin and fold change relative to Ox62+ DCs) of immunomodulatory molecules PDL-1, IDO (mean ± SEM *P ≤ 0.05, **P ≤ 0.01 and ***P ≤ 0.001 two-tailed Student's t-test n = 3). (d) DA BMDCs and Dexa BMDCs cocultured with allogeneic LEW lymphocytes have a reduced stimulatory capacity and an immunosuppressive capacity, illustrated by the reduction in percentage proliferating CFSE-labeled lymphocytes stimulated (10:1) with Ox62+ DC in the presence of (10:1 lymphocyte:BMDC) BMDCs or Dexa BMDCs (representative graph of four independent experiments mean ± SEM *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001 two-tailed Student's t-test). BMDC, bone marrow–derived dendritic cell; GM-CSF, granulocyte-macrophage colony-stimulating factor; LPS, lipopolysaccharide.
