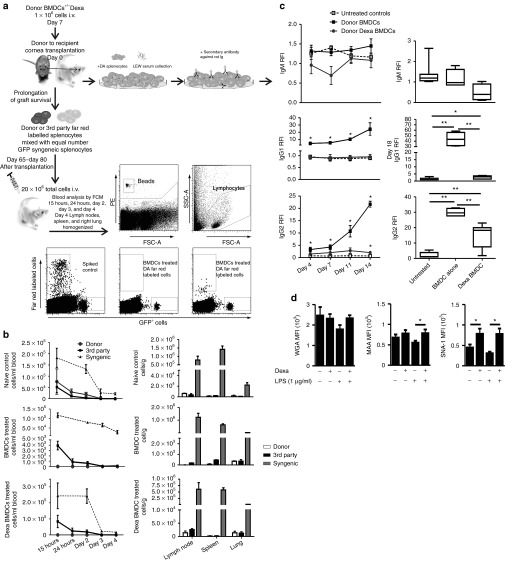
Figure 4.
Effects of donor untreated BMDC and Dexa BMDC administration on peripheral donor-specific unresponsiveness. (a) Illustration of experimental design for evaluation of peripheral donor-specific unresponsiveness (including gating strategy) and alloantibody detection. (b) Day 65–day 80 after transplantation, long-term allograft surviving and naïve ungrafted LEW rats were injected i.v. with a total of 20 × 106–labeled Far Red donor (DA) + GFP syngeneic (LEW) splenocytes or Far Red 3rd party (CD) + GFP syngeneic cells. Blood samples were collected from naïve ungrafted, BMDC- and Dexa BMDC–treated grafted groups 15 hours–day 4 after injections and analyzed for the detection of labeled cell populations. On day 4 after injection of labeled cells, LNs (submandibular, cervical, and deep cervical), spleen, and lung were also harvested (mean ± SEM n = 2–4 per group). (c) Differential levels of antidonor antibodies detectable in both BMDC- and Dexa BMDC–treated groups on day 4–day 14 after transplantation and day 18 (average time point of rejection) (mean ± SEM *P ≤ 0.05, **P ≤ 0.01 two-tailed Mann–Whitney test n = 3–7 per group). (d) Differences in the expression of cell surface glycans, N-acetylglucosamine, α2,3 linked sialic acids, and α2,6 sialic linked acids were analyzed using plant lectins WGA, MAA, and SNA-I (respectively), was also analyzed (mean ± SEM n = 3 experiments *P ≤ 0.05 one-tailed Student's t-test). BMDC, bone marrow–derived dendritic cell; MAA, Maackia amurensis; SNA-I, Sambucus nigra; WGA, wheat germ agglutinin.