Abstract
To maintain immunological balance the organism has to be tolerant to self while remaining competent to mount an effective immune response against third-party antigens. An important mechanism of this immune regulation involves the action of regulatory T-cell (Tregs). In this mini-review, we discuss some of the known and proposed mechanisms by which Tregs exert their influence in the context of immune regulation, and the contribution of mathematical modeling for these mechanistic studies. These models explore the mechanisms of action of regulatory T cells, and include hypotheses of multiple signals, delivered through simultaneous antigen-presenting cell (APC) conjugation; interaction of feedback loops between APC, Tregs, and effector cells; or production of specific cytokines that act on effector cells. As the field matures, and competing models are winnowed out, it is likely that we will be able to quantify how tolerance-inducing strategies, such as CD4-blockade, affect T-cell dynamics and what mechanisms explain the observed behavior of T-cell based tolerance.
Keywords: Tregs, mathematical models, CD4-blockade, regulation, tolerance
Introduction
Immunological tolerance can be defined as the state of unresponsiveness to an antigen, following prior contact with that antigen, where the host remains competent to mount an effective immune response against third-party antigens. Accomplishing therapeutic induced tolerance has been one of the major goals of immunology ever since the pioneering work of Medawar and colleagues (1).
There is a need to keep a balance between aggressive cells and cells that maintain tolerance to self. On occasions this balance can be disrupted originating either autoimmunity, when mechanisms leading to self-tolerance fail, or immunodeficiency and susceptibility to infection when the immune system is not able to mount a proper immune response. Usually, however, the immune system shows a significant capacity for self-tolerance, in spite of its equally efficient performance in the protection from foreign microbes. The ability to orchestrate protective immune responses is also the major hurdle impeding successful transplantation therapies and hinders the efficacy of therapeutic administration of foreign proteins and genes.
Random rearrangement of T-cell receptors (TCR) during cellular maturation leads to T cells that will recognize self-antigens. It was a long held assumption that central tolerance, by means of negative selection of autoreactive lymphocyte clones, could on its own account for the establishment of self-tolerance. Without such a censoring mechanism these autoreactive cells could eventually lead to autoimmune disease. Indeed thymocytes must survive the process of negative selection, which eliminates cells whose TCRs bind too avidly to self-antigens (2–4). The apoptosis of these thymocytes will prevent migration of autoreactive T cells to the periphery and prevent autoimmunity. Conversely, absence of negative selecting self-peptide-MHC complexes in the thymic medulla leads to an increase in mature autoreactive T cells (5, 6).
However, not all self-antigens are presented in the thymus, and some developing autoreactive T cells never encounter their antigens, eventually migrating to the periphery. Thus, although central tolerance contributes to the deletion of a large number of potentially autoreactive T cells, some autoreactive clones can be found in the periphery of healthy individuals (7, 8). There are, therefore, mechanisms that operate in the periphery (i.e., outside the thymus) to establish self-tolerance toward autoreactive clones that escape thymic negative selection.
Initially, one such mechanism was thought to be mediated by T-cell anergy, described as the functional state in which T cells remain viable but unable to respond to optimal stimulation through the TCR and co-stimulatory ligands (9), i.e., unable to proliferate or to produce interleukin-2 (IL-2) (10, 11). The first observation of anergy was made with purified human CD4+ T cells stimulated with large quantities of peptide antigens (10). It was noted that after antigen stimulation there was down-regulation of TCR and this was associated with the molecular mechanism for anergy (12). Subsequent studies with mouse CD4+ T cells suggested that occupancy of the TCR without any other signals was responsible for the induction of this state (13, 14).
Interestingly, anergic T cells were capable of suppressing proliferation of naïve T cells in vitro (15) and in vivo (16). In addition, anergic T cells have been shown to inhibit the antigen-presenting function and survival of dendritic cells (17). These and other observations led to the proposal of the “civil service model” (18), postulating that antigen-specific unresponsive cells can interfere with the generation of help by co-localizing with other T cells and competing for elements in the microenvironment (such as adhesion molecules or cytokines).
However, it was not clear how T cells would become anergic in vivo, and whether such mechanism was enough to maintain tolerance. More recently, a specific T cell subset, termed regulatory T (Treg) cells, gained prominence as being a key mechanism maintaining peripheral self-tolerance (19, 20). With hindsight, it is likely that many of the features of anergic T cells are a consequence of Treg function.
Regulatory T Cells
In 1995 Sakaguchi et al. (19) showed that depletion of a minor population of CD4+ T cells constitutively expressing CD25 [IL-2 receptor α-chain (IL-2Rα)] led to the generation of a spectrum of autoimmune diseases when transferred to immune-compromised recipients. In addition, the co-transfer of CD25+ T cells prevented the pathology.
Based on this CD25 marker, a population of natural (thymus-derived) regulatory T cells was identified in the resting immune system, both in mice and in humans (21). Subsequent studies showed that these cells express forkhead box transcription factor 3 (Foxp3) and this finding led to the definite establishment of a Treg subset (22–24). There is now abundant evidence that these regulatory T cells are actively engaged in the maintenance of self-tolerance (25). Furthermore, depletion of Foxp3+ Tregs originates fatal multi-organ autoimmunity. The phenotype of this disease is virtually indistinguishable from the IPEX syndrome, caused by Foxp3 mutations in humans and equivalent to the Scurfy phenotype in mice (26–28).
Thymic treg cells
The Treg cells that develop in the thymus, first described as naturally occurring regulatory T cells (nTregs) appear to be selected for self-antigen/MHC expressed by thymic epithelial cells (29, 30), in a process that requires TCR triggering in the presence of co-stimulation (31, 32), but dispenses TGF-β and IL-2 (33, 34). Early studies with Treg cells showed that these cells express CD25, CD5, and cytotoxic T lymphocyte antigen 4 (CTLA-4), which are all induced upon TCR stimulation (19).
In the periphery, nTregs represent around 6–10% of the overall CD4+ T-cell population. In order to be sustained they need continuous TCR triggering and co-stimulation in the presence of IL-2 (35–37), making IL-2 essential for natural Treg pool maintenance in the periphery (38). Comparative analysis of polyclonal TCR repertoires showed that TCR sequences from Treg cells were of broader variety and only partially overlapping with the ones from non-Treg cells (39). Some studies have shown that antigen-specific Treg cells are more potent at suppressing the induction of autoimmune disease than polyclonal populations (40). However, other studies have also shown that polyclonal Tregs are able to suppress independently of their specificity (41). Thus, Tregs with one antigen-specificity can suppress effector cells with many other antigen-specificities by bystander suppression. Moreover, transplantation studies have shown that Tregs can display a phenomenon called “linked suppression,” where they can be activated in an antigen-specific manner, and subsequently suppress responses to unrelated antigens presented by the same cells (42). Tregs show a third property called infectious tolerance by which one population of Treg cells creates a regulatory milieu that promotes the outgrowth of a new population of Treg cells with antigen-specificities distinct from those of the original population, as long as the new antigen is present in the same tissue as the antigen recognized by the original Treg cell (43–45).
Peripheral treg induction
Besides nTreg, of thymic origin, it has become apparent that induced regulatory T cells (iTreg) also exist in the periphery (46, 47). After the discovery of the key role for Foxp3 in Tregs, it was demonstrated that it was possible for non-Treg cells to acquire both Foxp3 and the regulatory functions associated with it, therefore becoming Treg cells themselves (46, 48, 49).
It is likely that peripheral induction of iTreg occurs in response to non-self antigens like food, allergens, and commensal bacteria (39). Early evidence for in vivo peripheral conversion was derived from adoptive cell transfer experiments in which polyclonal CD4+ CD25− naïve T cells were injected into lymphopenic mice or mice containing a monoclonal T cell repertoire devoid of nTregs, or when tolerance was imposed on monoclonal populations without Treg cells (49–51). In these conditions, homeostatic proliferation of the donor cells could be observed and part of the donor cell population became CD25+CTLA-4+GITR+Foxp3+ and acquired suppressive activity. Additionally, when congenitally marked CD4+ CD25− T cells were transferred to WT hosts, 10% of those converted into CD4+ CD25+ Foxp3+ T cells, within 6 weeks (52).
It was first shown in vitro that TCR activation in the presence of TGF-β would lead to Treg conversion (53). Subsequent studies supported this observation and demonstrated that iTreg conversion could be greatly enhanced by suboptimal TCR signals or a combination of strong TCR signals with high doses of TGF-β (47, 53–57). In vivo it is possible to induce oral tolerance by giving the antigen in the drinking water (58), or to induce transplantation tolerance using non-depleting anti-CD4 at the time of transplantation (48, 59). In both cases, tolerance induction is accompanied by induction of Foxp3+ cells, in a process that requires TGF-β. In addition to these, many other factors influence the induction of Tregs both in vitro and in vivo, such as the co-stimulation environment, the strength of TCR signaling, mTOR inhibition with rapamycin, and low levels of essential amino-acids (44, 57, 60–69).
Mechanisms of action of treg cells
In spite of intensive study of Tregs and their properties, the specific mechanisms by which they control immune responses are still not fully understood. There are several proposed mechanisms with experimental support, but it is likely that no single mechanism is responsible for the full range of biological phenomena involving Tregs (70). And it is also likely that in different milieu distinct mechanisms and even alternative subsets of regulatory cells are involved in tuning the immune response (71).
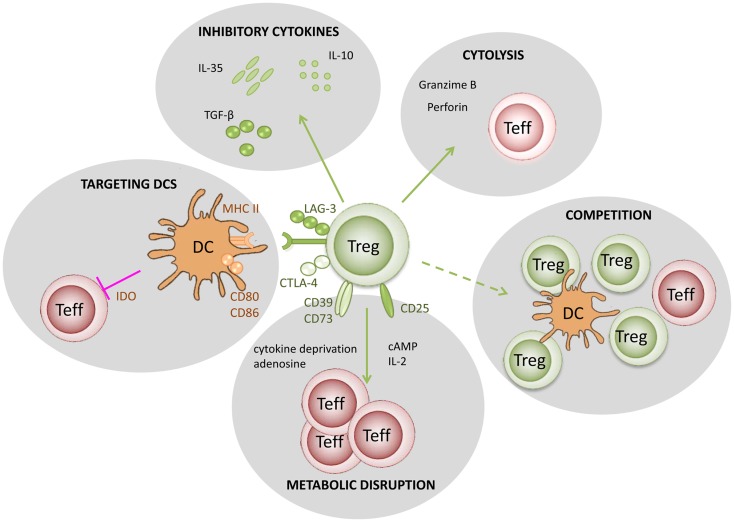
In Figure 1, we summarize five putative mechanisms of Treg function: (i) modulation of antigen-presenting cell (APC) activity through Treg engagement of co-stimulatory receptors on the surface of APC, leading to weak or abrogated signals from APC to naive/effector cells; (ii) Treg secretion of cytokines, such as IL10 and TGFβ, suppressing the activity of effector cells and APC; (iii) under certain circumstances, Tregs could have a direct cytotoxic effect, through the production of perforin/granzyme and induction of apoptosis in effector cells; (iv) Tregs may also cause metabolic disruption, for example stimulating APCs to produce enzymes that consume essential amino-acids, preventing naive/effector cell proliferation, and in the presence of TGFβ may induce the expression of Foxp3 in naive cells (i.e., they become Tregs); (v) Tregs could also compete with effectors cells for APC signals or cytokines, such as IL2.
Figure 1.
Putative mechanisms used by regulatory T cells. (1) Targeting DCs – modulation of antigen-presenting cell activity through Treg engagement of co-stimulatory receptors on the DC surface, leading to weak or abrogated signals to naïve/effector T cells; (2) Metabolic disruption – includes cytokine deprivation, cyclic AMP-mediated inhibition, and adenosine receptor (A2A)-mediated immunosuppression; (3) Competition – for critical cytokines, such as IL-2, or direct disruption of effector cell engagement with APCs; (4) Cytolysis – direct cytotoxic effect through the production of Granzyme B and Perforin and consequent apoptosis of effector T cells or APCs; (5) Production of inhibitory cytokines – including IL-10, IL-35, and TGF-β.
There is mounting evidence [reviewed in (72)] that Treg cells exert their effects on different cell types, including CD4+ and CD8+ T cells, B cells, natural killer T cells (NKT), and DCs (70). The action of Tregs can be mediated by secretion of immunosuppressive cytokines, such as IL-10, TGF-β, IL-35, and galectin-1 (72) or by cell-dependent mechanisms through molecules such as GITR, CTLA-4, CD39, CD73, and LAG-3 (70). The spectrum of effect of Tregs on their targets goes from modifying the functional properties of other immune cells, such as down-regulating transcription of IL-2 (70, 71, 73), and other important growth factors; to actually killing those cells through granzyme B and perforin (70, 73–77). For example, there is evidence that Tregs can kill both immature and mature DCs (74).
Furthermore, Tregs may convert APCs to become themselves immunosuppressive (78). It has also been proposed that Tregs act by competing with other cells for growth factors, particularly IL-2 (79, 80). One possible outcome of these interactions is that other cells become themselves Foxp3+ regulatory cells (45).
These and other suppressive mechanisms may be operational depending on the microenvironment, biological context, and immune response. For instance, IL-10 producing cells are more abundant in lamina propria (81, 82) and perforin or granzyme expressing Tregs are predominant in tumor environments (83).
Mathematical Modeling
Due to the complexity of the mechanisms and interactions involved in the processes of immune tolerance, mathematical modeling has been used as a tool to explore different conceptual frameworks of immunological tolerance. Many studies have analyzed the dynamics of thymocyte development, with positive and negative selection, as a mechanism of central tolerance (84–91).
Many other studies have focused on modeling the putative mechanisms of Treg suppression in the periphery. In these models, typically the dynamics of Tregs, effector cells, and APCs are studied to find the interaction mechanisms in the model that qualitatively reflect the experimental knowledge. For the purposes of this review, we can divide the models proposed in three categories, although there is overlap between these in some studies: (i) models that analyze different putative mechanisms of action of Tregs (Table 1); (ii) models that analyze the effects of Tregs on different processes, such as the immune response to pathogens and tumors, or in allergy; and (iii) models that study the maintenance of Tregs (homeostasis).
Table 1.
Summary of mechanistic models of Treg action.
| Cell populations considered | Mechanisms of regulation of immune response | Some properties of the model | Reference |
|---|---|---|---|
| APC, Treg, Teff, and Treg, Teff conjugates on APC | Competition for activation on APC Tregs inhibit Teff on same conjugate Treg maintenance is dependent on Teff | Treg inhibit growth of Teff Treg induce Teff to become Treg | (92–94, 96) |
| No explicit APC dynamics | |||
| As above plus IL2 | Competition for IL2 | Non-local interactions | (97, 98) |
| Tregs condition APC | Model used to study IL2-based therapies | ||
| APC and Ag dynamics | Tregs directly suppress Teff (specifically and bystander) | Bystander effects are important | (99) |
| APC maturation | Tregs suppress APC maturation | Direct suppression was more effective | |
| T cells are activated into Treg or Teff by APC stimulation | |||
| Antigen | Tp become Treg by interaction with resting APC | Strength of antigen stimulus is crucial in defining whether system is in tolerant or non-tolerant state | (100) |
| Immature APC, resting APC, activated APC | Tp become Teff by interaction with activated APC Teff activates APC Treg induces activated APC to rest | ||
| Precursor T cells (Tp), Teff, Treg | |||
| Stochastic model of TCR triggering for T cells (both thymus and periphery) | Different thresholds for activation vs. anergy, with or without co-stimulation | Self-reactive cells in periphery are controlled by a mechanism of reversible anergy | (103) |
| T cells with tunable activation thresholds | Model for integration of signals in successive encounters with APC | Exhibits self-tolerance | (102) |
| “More cells should lead to less anergy,” which is not seen in adoptive transfer experiments | |||
| Inactive and active Treg and Teff | Tregs consume IL2 | Strength of antigen stimulation (for Treg and Teff) defines relative levels of those two populations | (107–109) |
| IL2 for Teff proliferation, also helps Treg proliferate | Treg inhibit Teff (from active to inactive) proportionally to Treg numbers | ||
| Cytokine (e.g., IL7) for Treg homeostasis | |||
| APC with different antigens Teff of multiple specificities Tregs of multiple specificities | Cells interact with extensive cross-reactivity, but different avidities | Effector functions are the outcome of individual cellular decisions (based on cross-reactivity) | (111) |
| A threshold of conjugation time can be identified that permits self/non-self discrimination |
Comparison of some of the models for Treg action discussed in the text. The model by Kim et al. (110) is too complex to fit in this summary table.
Models of the mechanisms of treg action
An early model explicitly considering Tregs was developed by León and collaborators (92). They considered cross-regulation, where simultaneous conjugation of a Treg and an effector cell on the same APC can suppress effector function (92, 93). In this model, regulation could be due to competition for conjugation sites on the APC, or through inhibitory signals delivered to effector cells on the same APC, or by inducing conversion of effector cells to a regulatory phenotype. This model is developed and analyzed in detail in several subsequent publications (93–95), and it is reviewed in Carneiro et al. (96). Recently the model was expanded to study the dual effect of IL2 in promoting immunity and tolerance (97, 98). Some authors considered in more detail the dynamics of antigen and APCs, and compared mechanisms where regulatory T cells suppress APCs function or maturation with models where Tregs act directly on effector T cells (99). Other models along these lines included the processes of APC maturation and the differentiation of T cells into regulatory or effector phenotypes (100), following a previous proposal for this interaction (101). Interestingly, in these models, survival or proliferation of Tregs is dependent on feedback from effector T-cells, which is in part responsible for the bi-stability observed that is interpreted as states of tolerance or immunity.
Another mechanism of peripheral tolerance modeled by several authors involves anergy of effector cells (102, 103). This anergy can be achieved by tuning the threshold for activation, for example through repeated encounter with antigen or APC (102–106), or through modulation by Tregs. Carneiro et al. compared this mechanism with their previous model of cross-regulation discussed above (102). Another model that also explores thresholds for activation, but based on effector T-cell population response was studied by Burroughs et al. (107, 108). In this model the relative levels of Tregs and effector T cells depend on the respective strength of stimulation by antigen, which can be modulated by IL-2 – this model is reviewed in (109).
Typically these models consider a limited number of cell populations (3–6) and analyze one mechanism at a time. However, Kim et al. proposed a detailed model including dozens of cell populations, with a spatial component (tissue and lymph node), and considered multiple mechanisms of Treg action simultaneously (110). At the other end of the spectrum, Abreu et al. proposed a model where regulation of the immune system was simply based on cross-recognition of multiple antigens by the same cell, whether it is an effector, a regulatory, or an APC (111).
Models of the effect of tregs on the immune response
The studies discussed so far are mainly concerned with the mechanisms defining the interactions of Tregs and effector cells, often looking for steady states where one or the other population dominates, interpreted as tolerance or autoimmune states. Other models analyze the effects of the existence of Tregs on different processes.
Many of these models explore the system level effects of Treg failure and the potential development of autoimmunity. One of the first models to study this was by León et al. (112), where they analyzed the relationship between infections and autoimmunity in general. More recent studies analyzed specific autoimmune conditions, such as multiple sclerosis (113, 114) and inflammatory bowel disease (115). Grosse et al. analyzed the balance of Th1 vs. Th2 type responses and their control by Tregs in the context of allergies, with the objective of analyzing immunotherapy protocols (116). Another study looking at immunotherapy protocols, in this case modulation of IL2 therapy, used a mathematical model of helper, regulatory, and memory CD4+ T cells (98). These studies are mostly theoretical. However, one report described an interesting experimental study of mice injected with tolerogenic or control peptides and followed for 16 days, with serial measurements of different T-cell subsets. These data were then analyzed with a mathematical model (117).
Some studies have analyzed the interplay between infections and regulation of the immune response. One of these modeled the immune decision between attacking or not a given antigen, based on the network of interactions between Tregs, Th17 cells, and growing levels of antigen (as in the case of a pathogen) (118). And a model analyzing the regulation of the immune response in early HIV infection, through the expansion of specific Tregs, was recently introduced (119). Finally, León et al. (120, 121) considered an expansion of their mechanistic model of Treg – T effector interactions to study the immune response against tumors, and their control or expansion.
Models of treg homeostasis
An important question that has also been addressed by modeling studies is the maintenance of a healthy number of Tregs. Several studies included the possibility of the Treg population being maintained in the periphery in part by feedback from the effector cells (92, 99, 100). One such model (94) studied the effects of thymic output and positive/negative selection on proper balance between tolerance and immunity in the periphery, and concluded that repertoire selection plays an important role in maintaining that balance. Baltcheva et al. (122) developed a more detailed model to analyze the life-long dynamics of precursor and mature CD25+ T cells (Tregs) in humans, including thymic production, density-dependent homeostasis, and effector T-cell conversion.
Conclusion
The field of regulatory T cells, although relatively recent, has had an explosion of knowledge driven by detailed experimental work (20, 65, 72, 123, 124). Indeed there are many more studies than we could possibly review or even allude to in this mini-review. However, the mechanistic details of this important function of the immune system are not completely elucidated (72). Many authors have developed mathematical models of the interactions between Tregs and effector cells to try to add to our understanding of these mechanisms. Still, there is a lack of true collaborations between experimental scientists and modelers in this field. Clearly, more progress would be possible if such integrated teams worked together, as has been the case in other areas of medicine, e.g., modeling of viral infections (125). As the field matures and competing models are winnowed out, it is likely that we will be able to quantify how tolerance-inducing strategies, such as CD4-blockade, affect T-cell dynamics, and what mechanisms explain the observed behavior of T-cell based tolerance.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Joana Duarte for assistance in preparing Figure 1. Luis Graca is funded by FCT/PTDC/SAU-TOX/114424/2009 and FCT/PTDC/SAU-IMU/120225/2010. Ruy M. Ribeiro received funding from the European Union 7th Framework Programme under grant n° PCOFUND-GA-2009-246542 and from Fundação para a Ciência e Tecnologia, Portugal.
References
- 1.Medawar PB. The behaviour and fate of skin autografts and skin homografts in rabbits: a report to the War Wounds Committee of the Medical Research Council. J Anat (1944) 78(Pt 5):176–99Epub 1944/10/01 [PMC free article] [PubMed] [Google Scholar]
- 2.Kappler JW, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell (1987) 49(2):273–80 Epub 1987/04/24 10.1016/0092-8674(87)90568-X [DOI] [PubMed] [Google Scholar]
- 3.MacDonald HR, Schneider R, Lees RK, Howe RC, Acha-Orbea H, Festenstein H, et al. T-cell receptor V beta use predicts reactivity and tolerance to Mlsa-encoded antigens. Nature (1988) 332(6159):40–5 Epub 1988/03/03 10.1038/332040a0 [DOI] [PubMed] [Google Scholar]
- 4.von Boehmer H, Teh HS, Kisielow P. The thymus selects the useful, neglects the useless and destroys the harmful. Immunol Today (1989) 10(2):57–61 Epub 1989/02/01 10.1016/0167-5699(89)90307-1 [DOI] [PubMed] [Google Scholar]
- 5.Laufer TM, DeKoning J, Markowitz JS, Lo D, Glimcher LH. Unopposed positive selection and autoreactivity in mice expressing class II MHC only on thymic cortex. Nature (1996) 383(6595):81–5 Epub 1996/09/05 10.1038/383081a0 [DOI] [PubMed] [Google Scholar]
- 6.Laufer TM, Fan L, Glimcher LH. Self-reactive T cells selected on thymic cortical epithelium are polyclonal and are pathogenic in vivo. J Immunol (1999) 162(9):5078–84Epub 1999/05/05 [PubMed] [Google Scholar]
- 7.Ramsdell F, Lantz T, Fowlkes BJ. A nondeletional mechanism of thymic self tolerance. Science (1989) 246(4933):1038–41 Epub 1989/11/24 10.1126/science.2511629 [DOI] [PubMed] [Google Scholar]
- 8.Schonrich G, Momburg F, Hammerling GJ, Arnold B. Anergy induced by thymic medullary epithelium. Eur J Immunol (1992) 22(7):1687–91 Epub 1992/07/01 10.1002/eji.1830220704 [DOI] [PubMed] [Google Scholar]
- 9.Schwartz RH. Models of T cell anergy: is there a common molecular mechanism? J Exp Med (1996) 184(1):1–8 Epub 1996/07/01 10.1084/jem.184.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lamb JR, Skidmore BJ, Green N, Chiller JM, Feldmann M. Induction of tolerance in influenza virus-immune T lymphocyte clones with synthetic peptides of influenza hemagglutinin. J Exp Med (1983) 157(5):1434–47 Epub 1983/05/01 10.1084/jem.157.5.1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz RH. A cell culture model for T lymphocyte clonal anergy. Science (1990) 248(4961):1349–56 Epub 1990/06/15 10.1126/science.2113314 [DOI] [PubMed] [Google Scholar]
- 12.Lamb JR, Feldmann M. Essential requirement for major histocompatibility complex recognition in T-cell tolerance induction. Nature (1984) 308(5954):72–4 Epub 1984/03/01 10.1038/308072a0 [DOI] [PubMed] [Google Scholar]
- 13.Jenkins MK, Schwartz RH. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J Exp Med (1987) 165(2):302–19 Epub 1987/02/01 10.1084/jem.165.2.302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quill H, Schwartz RH. Stimulation of normal inducer T cell clones with antigen presented by purified Ia molecules in planar lipid membranes: specific induction of a long-lived state of proliferative nonresponsiveness. J Immunol (1987) 138(11):3704–12Epub 1987/06/01 [PubMed] [Google Scholar]
- 15.Lombardi G, Sidhu S, Batchelor R, Lechler R. Anergic T cells as suppressor cells in vitro. Science (1994) 264(5165):1587–9 Epub 1994/06/10 10.1126/science.8202711 [DOI] [PubMed] [Google Scholar]
- 16.Chai JG, Bartok I, Chandler P, Vendetti S, Antoniou A, Dyson J, et al. Anergic T cells act as suppressor cells in vitro and in vivo. Eur J Immunol (1999) 29(2):686–92 Epub 1999/03/04 [DOI] [PubMed] [Google Scholar]
- 17.Vendetti S, Chai JG, Dyson J, Simpson E, Lombardi G, Lechler R. Anergic T cells inhibit the antigen-presenting function of dendritic cells. J Immunol (2000) 165(3):1175–81Epub 2000/07/21 [DOI] [PubMed] [Google Scholar]
- 18.Waldmann H, Qin S, Cobbold S. Monoclonal antibodies as agents to reinduce tolerance in autoimmunity. J Autoimmun (1992) 5(Suppl A):93–102 Epub 1992/04/01 10.1016/0896-8411(92)90024-K [DOI] [PubMed] [Google Scholar]
- 19.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol (1995) 155(3):1151–64Epub 1995/08/01 [PubMed] [Google Scholar]
- 20.Ohkura N, Kitagawa Y, Sakaguchi S. Development and maintenance of regulatory T cells. Immunity (2013) 38(3):414–23 10.1016/j.immuni.2013.03.002 [DOI] [PubMed] [Google Scholar]
- 21.Yagi H, Nomura T, Nakamura K, Yamazaki S, Kitawaki T, Hori S, et al. Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int Immunol (2004) 16(11):1643–56 10.1093/intimm/dxh165 [DOI] [PubMed] [Google Scholar]
- 22.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol (2003) 4(4):330–6 10.1038/ni904 [DOI] [PubMed] [Google Scholar]
- 23.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science (2003) 299(5609):1057–61 Epub 2003/01/11 10.1126/science.1079490 [DOI] [PubMed] [Google Scholar]
- 24.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol (2003) 4(4):337–42 10.1038/ni909 [DOI] [PubMed] [Google Scholar]
- 25.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol (2004) 22:531–62 Epub 2004/03/23 10.1146/annurev.immunol.21.120601.141122 [DOI] [PubMed] [Google Scholar]
- 26.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet (2001) 27(1):20–1 Epub 2001/01/04 10.1038/83713 [DOI] [PubMed] [Google Scholar]
- 27.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet (2001) 27(1):18–20 Epub 2001/01/04 10.1038/83707 [DOI] [PubMed] [Google Scholar]
- 28.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet (2001) 27(1):68–73 10.1038/83784 [DOI] [PubMed] [Google Scholar]
- 29.Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immunol (2002) 3(8):756–63 10.1038/ni816 [DOI] [PubMed] [Google Scholar]
- 30.Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, et al. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol (2001) 2(4):301–6 Epub 2001/03/29 10.1038/86302 [DOI] [PubMed] [Google Scholar]
- 31.Tai X, Cowan M, Feigenbaum L, Singer A. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat Immunol (2005) 6(2):152–62 Epub 2005/01/11 10.1038/ni1160 [DOI] [PubMed] [Google Scholar]
- 32.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity (2005) 22(3):329–41 10.1016/j.immuni.2005.01.016 [DOI] [PubMed] [Google Scholar]
- 33.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol (2005) 6(11):1142–51 10.1038/ni1263 [DOI] [PubMed] [Google Scholar]
- 34.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med (2005) 201(7):1061–7 10.1084/jem.20042276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gavin MA, Clarke SR, Negrou E, Gallegos A, Rudensky A. Homeostasis and anergy of CD4(+)CD25(+) suppressor T cells in vivo. Nat Immunol (2002) 3(1):33–41 10.1038/ni743 [DOI] [PubMed] [Google Scholar]
- 36.Knoechel B, Lohr J, Kahn E, Bluestone JA, Abbas AK. Sequential development of interleukin 2-dependent effector and regulatory T cells in response to endogenous systemic antigen. J Exp Med (2005) 202(10):1375–86 10.1084/jem.20050855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lohr J, Knoechel B, Jiang S, Sharpe AH, Abbas AK. The inhibitory function of B7 costimulators in T cell responses to foreign and self-antigens. Nat Immunol (2003) 4(7):664–9 10.1038/ni939 [DOI] [PubMed] [Google Scholar]
- 38.Malek TR. The main function of IL-2 is to promote the development of T regulatory cells. J Leukoc Biol (2003) 74(6):961–5 10.1189/jlb.0603272 [DOI] [PubMed] [Google Scholar]
- 39.Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol (2012) 30:531–64 Epub 2012/01/10 10.1146/annurev.immunol.25.022106.141623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity (2000) 12(4):431–40 Epub 2000/05/05 10.1016/S1074-7613(00)80195-8 [DOI] [PubMed] [Google Scholar]
- 41.Graca L, Le Moine A, Lin CY, Fairchild PJ, Cobbold SP, Waldmann H. Donor-specific transplantation tolerance: the paradoxical behavior of CD4+CD25+ T cells. Proc Natl Acad Sci U S A (2004) 101(27):10122–6 Epub 2004/06/26 10.1073/pnas.0400084101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davies JD, Leong LY, Mellor A, Cobbold SP, Waldmann H. T cell suppression in transplantation tolerance through linked recognition. J Immunol (1996) 156(10):3602–7Epub 1996/05/15 [PubMed] [Google Scholar]
- 43.Qin S, Cobbold SP, Pope H, Elliott J, Kioussis D, Davies J, et al. “Infectious” transplantation tolerance. Science (1993) 259(5097):974–7Epub 1993/02/12 [DOI] [PubMed] [Google Scholar]
- 44.Graca L, Honey K, Adams E, Cobbold SP, Waldmann H. Cutting edge: anti-CD154 therapeutic antibodies induce infectious transplantation tolerance. J Immunol (2000) 165(9):4783–6Epub 2000/10/25 [DOI] [PubMed] [Google Scholar]
- 45.Kendal AR, Chen Y, Regateiro FS, Ma J, Adams E, Cobbold SP, et al. Sustained suppression by Foxp3+ regulatory T cells is vital for infectious transplantation tolerance. J Exp Med (2011) 208(10):2043–53 Epub 2011/08/31 10.1084/jem.20110767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Apostolou I, von Boehmer H. In vivo instruction of suppressor commitment in naive T cells. J Exp Med (2004) 199(10):1401–8 Epub 2004/05/19 10.1084/jem.20040249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol (2005) 6(12):1219–27 Epub 2005/10/26 10.1038/ni1265 [DOI] [PubMed] [Google Scholar]
- 48.Cobbold SP, Castejon R, Adams E, Zelenika D, Graca L, Humm S, et al. Induction of foxP3+ regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants. J Immunol (2004) 172(10):6003–10Epub 2004/05/07 [DOI] [PubMed] [Google Scholar]
- 49.Curotto de Lafaille MA, Lino AC, Kutchukhidze N, Lafaille JJ. CD25− T cells generate CD25+Foxp3+ regulatory T cells by peripheral expansion. J Immunol (2004) 173(12):7259–68 [DOI] [PubMed] [Google Scholar]
- 50.Furtado GC, Curotto de Lafaille MA, Kutchukhidze N, Lafaille JJ. Interleukin 2 signaling is required for CD4(+) regulatory T cell function. J Exp Med (2002) 196(6):851–7 10.1084/jem.20020190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cobbold SP, Graca L, Lin CY, Adams E, Waldmann H. Regulatory T cells in the induction and maintenance of peripheral transplantation tolerance. Transpl Int (2003) 16(2):66–75 Epub 2003/02/22 10.1111/j.1432-2277.2003.tb00266.x [DOI] [PubMed] [Google Scholar]
- 52.Liang S, Alard P, Zhao Y, Parnell S, Clark SL, Kosiewicz MM. Conversion of CD4+ CD25− cells into CD4+ CD25+ regulatory T cells in vivo requires B7 costimulation, but not the thymus. J Exp Med (2005) 201(1):127–37 10.1084/jem.20041201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med (2003) 198(12):1875–86 10.1084/jem.20030152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Selvaraj RK, Geiger TL. A kinetic and dynamic analysis of Foxp3 induced in T cells by TGF-beta. J Immunol (2007) 178(12):7667–77 [DOI] [PubMed] [Google Scholar]
- 55.Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA. Natural and induced CD4+CD25+ cells educate CD4+CD25− cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10. J Immunol (2004) 172(9):5213–21 [DOI] [PubMed] [Google Scholar]
- 56.Graca L, Chen TC, Le Moine A, Cobbold SP, Howie D, Waldmann H. Dominant tolerance: activation thresholds for peripheral generation of regulatory T cells. Trends Immunol (2005) 26(3):130–5 Epub 2005/03/05 10.1016/j.it.2004.12.007 [DOI] [PubMed] [Google Scholar]
- 57.Oliveira VG, Caridade M, Paiva RS, Demengeot J, Graca L. Sub-optimal CD4+ T-cell activation triggers autonomous TGF-beta-dependent conversion to Foxp3+ regulatory T cells. Eur J Immunol (2011) 41(5):1249–55 Epub 2011/04/07 10.1002/eji.201040896 [DOI] [PubMed] [Google Scholar]
- 58.Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ. Curotto de Lafaille MA. Oral tolerance in the absence of naturally occurring Tregs. J Clin Invest (2005) 115(7):1923–33 Epub 2005/06/07 10.1172/JCI24487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin CY, Graca L, Cobbold SP, Waldmann H. Dominant transplantation tolerance impairs CD8+ T cell function but not expansion. Nat Immunol (2002) 3(12):1208–13 Epub 2002/11/05 10.1038/ni853 [DOI] [PubMed] [Google Scholar]
- 60.Chen TC, Waldmann H, Fairchild PJ. Induction of dominant transplantation tolerance by an altered peptide ligand of the male antigen Dby. J Clin Invest (2004) 113(12):1754–62 Epub 2004/06/17 10.1172/JCI20569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Griffin MD, Lutz W, Phan VA, Bachman LA, McKean DJ, Kumar R. Dendritic cell modulation by 1alpha,25 dihydroxyvitamin D3 and its analogs: a vitamin D receptor-dependent pathway that promotes a persistent state of immaturity in vitro and in vivo. Proc Natl Acad Sci U S A (2001) 98(12):6800–5 Epub 2001/05/24 10.1073/pnas.121172198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Manicassamy S, Pulendran B. Dendritic cell control of tolerogenic responses. Immunol Rev (2011) 241(1):206–27 Epub 2011/04/15 10.1111/j.1600-065X.2011.01015.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science (2007) 317(5835):256–60 Epub 2007/06/16 10.1126/science.1145697 [DOI] [PubMed] [Google Scholar]
- 64.Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol (1997) 159(10):4772–80Epub 1997/11/20 [PubMed] [Google Scholar]
- 65.Waldmann H, Chen TC, Graca L, Adams E, Daley S, Cobbold S, et al. Regulatory T cells in transplantation. Semin Immunol (2006) 18(2):111–9 Epub 2006/02/16 10.1016/j.smim.2006.01.010 [DOI] [PubMed] [Google Scholar]
- 66.Yang J, Bernier SM, Ichim TE, Li M, Xia X, Zhou D, et al. LF15-0195 generates tolerogenic dendritic cells by suppression of NF-kappaB signaling through inhibition of IKK activity. J Leukoc Biol (2003) 74(3):438–47 Epub 2003/09/02 10.1189/jlb.1102582 [DOI] [PubMed] [Google Scholar]
- 67.Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol (2006) 177(12):8338–47 [DOI] [PubMed] [Google Scholar]
- 68.Cobbold SP, Adams E, Farquhar CA, Nolan KF, Howie D, Lui KO, et al. Infectious tolerance via the consumption of essential amino acids and mTOR signaling. Proc Natl Acad Sci U S A (2009) 106(29):12055–60 10.1073/pnas.0903919106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang R, Huynh A, Whitcher G, Chang J, Maltzman JS, Turka LA. An obligate cell-intrinsic function for CD28 in Tregs. J Clin Invest (2013) 123(2):580–93 10.1172/JCI65013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol Med (2007) 13(3):108–16 Epub 2007/01/30 10.1016/j.molmed.2007.01.003 [DOI] [PubMed] [Google Scholar]
- 71.Toda A, Piccirillo CA. Development and function of naturally occurring CD4+CD25+ regulatory T cells. J Leukoc Biol (2006) 80(3):458–70 Epub 2006/07/01 10.1189/jlb.0206095 [DOI] [PubMed] [Google Scholar]
- 72.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity (2009) 30(5):636–45 10.1016/j.immuni.2009.04.010 [DOI] [PubMed] [Google Scholar]
- 73.Wing K, Fehervari Z, Sakaguchi S. Emerging possibilities in the development and function of regulatory T cells. Int Immunol (2006) 18(7):991–1000 Epub 2006/05/25 10.1093/intimm/dxl044 [DOI] [PubMed] [Google Scholar]
- 74.Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity (2004) 21(4):589–601 Epub 2004/10/16 10.1016/j.immuni.2004.09.002 [DOI] [PubMed] [Google Scholar]
- 75.Gondek DC, Lu LF, Quezada SA, Sakaguchi S, Noelle RJ. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol (2005) 174(4):1783–6Epub 2005/02/09. [DOI] [PubMed] [Google Scholar]
- 76.Sakaguchi S, Wing K, Onishi Y, Prieto-Martin P, Yamaguchi T. Regulatory T cells: how do they suppress immune responses? Int Immunol (2009) 21(10):1105–11 Epub 2009/09/10 10.1093/intimm/dxp095 [DOI] [PubMed] [Google Scholar]
- 77.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol (2008) 8(7):523–32 10.1038/nri2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kryczek I, Wei S, Zou L, Zhu G, Mottram P, Xu H, et al. Cutting edge: induction of B7-H4 on APCs through IL-10: novel suppressive mode for regulatory T cells. J Immunol (2006) 177(1):40–4Epub 2006/06/21 [DOI] [PubMed] [Google Scholar]
- 79.Scheffold A, Huhn J, Hofer T. Regulation of CD4+CD25+ regulatory T cell activity: it takes (IL-)two to tango. Eur J Immunol (2005) 35(5):1336–41 Epub 2005/04/14 10.1002/eji.200425887 [DOI] [PubMed] [Google Scholar]
- 80.Scheffold A, Murphy KM, Hofer T. Competition for cytokines: T(reg) cells take all. Nat Immunol (2007) 8(12):1285–7 Epub 2007/11/21 10.1038/ni1207-1285 [DOI] [PubMed] [Google Scholar]
- 81.Maynard CL, Harrington LE, Janowski KM, Oliver JR, Zindl CL, Rudensky AY, et al. Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3− precursor cells in the absence of interleukin 10. Nat Immunol (2007) 8(9):931–41 10.1038/ni1504 [DOI] [PubMed] [Google Scholar]
- 82.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity (2008) 28(4):546–58 10.1016/j.immuni.2008.02.017 [DOI] [PubMed] [Google Scholar]
- 83.Cao X, Cai SF, Fehniger TA, Song J, Collins LI, Piwnica-Worms DR, et al. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity (2007) 27(4):635–46 10.1016/j.immuni.2007.08.014 [DOI] [PubMed] [Google Scholar]
- 84.Faro J, Velasco S, Gonzalez-Fernandez A, Bandeira A. The impact of thymic antigen diversity on the size of the selected T cell repertoire. J Immunol (2004) 172(4):2247–55 [DOI] [PubMed] [Google Scholar]
- 85.Detours V, Mehr R, Perelson AS. Deriving quantitative constraints on T cell selection from data on the mature T cell repertoire. J Immunol (2000) 164(1):121–8 [DOI] [PubMed] [Google Scholar]
- 86.Detours V, Perelson AS. Explaining high alloreactivity as a quantitative consequence of affinity-driven thymocyte selection. Proc Natl Acad Sci U S A (1999) 96(9):5153–8 10.1073/pnas.96.9.5153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mehr R, Globerson A, Perelson AS. Modeling positive and negative selection and differentiation processes in the thymus. J Theor Biol (1995) 175(1):103–26 10.1006/jtbi.1995.0124 [DOI] [PubMed] [Google Scholar]
- 88.Currie J, Castro M, Lythe G, Palmer E, Molina-Paris C. A stochastic T cell response criterion. J R Soc Interface (2012) 9(76):2856–70 10.1098/rsif.2012.0205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Castiglione F, Santoni D, Rapin N. CTLs’ repertoire shaping in the thymus: a Monte Carlo simulation. Autoimmunity (2011) 44(4):261–70 10.3109/08916934.2011.523272 [DOI] [PubMed] [Google Scholar]
- 90.Muller V, Bonhoeffer S. Quantitative constraints on the scope of negative selection. Trends Immunol (2003) 24(3):132–5 10.1016/S1471-4906(03)00028-0 [DOI] [PubMed] [Google Scholar]
- 91.Sawicka ML, Reynolds J, Abourashchi N, Lythe G, Molina-Paris C, et al. Immunology and mathematics: a joint effort to estimate (murine) thymic selection rates. Front Immunol (in press).
- 92.Leon K, Perez R, Lage A, Carneiro J. Modelling T-cell-mediated suppression dependent on interactions in multicellular conjugates. J Theor Biol (2000) 207(2):231–54 Epub 2000/10/18 10.1006/jtbi.2000.2169 [DOI] [PubMed] [Google Scholar]
- 93.Leon K, Perez R, Lage A, Carneiro J. Three-cell interactions in T cell-mediated suppression? A mathematical analysis of its quantitative implications. J Immunol (2001) 166(9):5356–65Epub 2001/04/21 [DOI] [PubMed] [Google Scholar]
- 94.Leon K, Lage A, Carneiro J. Tolerance and immunity in a mathematical model of T-cell mediated suppression. J Theor Biol (2003) 225(1):107–26 Epub 2003/10/16 10.1016/S0022-5193(03)00226-1 [DOI] [PubMed] [Google Scholar]
- 95.Leon K, Faro J, Carneiro J. A general mathematical framework to model generation structure in a population of asynchronously dividing cells. J Theor Biol (2004) 229(4):455–76 Epub 2004/07/13 10.1016/j.jtbi.2004.04.011 [DOI] [PubMed] [Google Scholar]
- 96.Carneiro J, Leon K, Caramalho I, van den Dool C, Gardner R, Oliveira V, et al. When three is not a crowd: a crossregulation model of the dynamics and repertoire selection of regulatory CD4+ T cells. Immunol Rev (2007) 216:48–68 Epub 2007/03/21 10.1111/j.1600-065X.2007.00487.x [DOI] [PubMed] [Google Scholar]
- 97.Garcia-Martinez K, Leon K. Modeling the role of IL-2 in the interplay between CD4+ helper and regulatory T cells: assessing general dynamical properties. J Theor Biol (2010) 262(4):720–32 Epub 2009/11/03 10.1016/j.jtbi.2009.10.025 [DOI] [PubMed] [Google Scholar]
- 98.Garcia-Martinez K, Leon K. Modeling the role of IL2 in the interplay between CD4+ helper and regulatory T cells: studying the impact of IL2 modulation therapies. Int Immunol (2012) 24(7):427–46 10.1093/intimm/dxr120 [DOI] [PubMed] [Google Scholar]
- 99.Alexander HK, Wahl LM. Self-tolerance and autoimmunity in a regulatory T cell model. Bull Math Biol (2011) 73(1):33–71 Epub 2010/03/03 10.1007/s11538-010-9519-2 [DOI] [PubMed] [Google Scholar]
- 100.Fouchet D, Regoes R. A population dynamics analysis of the interaction between adaptive regulatory T cells and antigen presenting cells. PLoS One (2008) 3(5):e2306 Epub 2008/05/30 10.1371/journal.pone.0002306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Powrie F, Maloy KJ. Immunology. regulating the regulators. Science (2003) 299(5609):1030–1 10.1126/science.1082031 [DOI] [PubMed] [Google Scholar]
- 102.Carneiro J, Paixao T, Milutinovic D, Sousa J, Leon K, Gardner R, et al. Immunological self-tolerance: Lessons from mathematical modeling. J Comput Appl Math (2005) 184(1):77–100 10.1016/j.cam.2004.10.025 [DOI] [Google Scholar]
- 103.Chan C, Stark J, George AJT. The impact of multiple T cell-APC encounters and the role of anergy. J Comput Appl Math (2005) 184(1):101–20 10.1016/j.cam.2004.07.036 [DOI] [Google Scholar]
- 104.Anderton SM, Wraith DC. Selection and fine-tuning of the autoimmune T-cell repertoire. Nat Rev Immunol (2002) 2(7):487–98 10.1038/nri842 [DOI] [PubMed] [Google Scholar]
- 105.Grossman Z, Paul WE. Adaptive cellular interactions in the immune system: the tunable activation threshold and the significance of subthreshold responses. Proc Natl Acad Sci U S A (1992) 89(21):10365–9 10.1073/pnas.89.21.10365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.van den Berg HA, Rand DA. Quantitative theories of T-cell responsiveness. Immunol Rev (2007) 216:81–92 10.1111/j.1600-065X.2006.00491.x [DOI] [PubMed] [Google Scholar]
- 107.Burroughs NJ, Miguel Paz Mendes de Oliveira B, Adrego Pinto A. Regulatory T cell adjustment of quorum growth thresholds and the control of local immune responses. J Theor Biol (2006) 241(1):134–41 Epub 2006/01/13 10.1016/j.jtbi.2005.11.010 [DOI] [PubMed] [Google Scholar]
- 108.Burroughs NJ, Oliveira BM, Pinto AA, Sequeira HJT. Sensibility of the quorum growth thresholds controlling local immune responses. Math Comput Model (2008) 47(7–8):714–25 10.1016/j.mcm.2007.06.007 [DOI] [Google Scholar]
- 109.Pinto AA, Burroughs NJ, Ferreira M, Oliveira BM. Dynamics of immunological models. Acta Biotheor (2010) 58(4):391–404 10.1007/s10441-010-9117-6 [DOI] [PubMed] [Google Scholar]
- 110.Kim PS, Lee PP, Levy D. Modeling regulation mechanisms in the immune system. J Theor Biol (2007) 246(1):33–69 Epub 2007/02/03 10.1016/j.jtbi.2006.12.012 [DOI] [PubMed] [Google Scholar]
- 111.de Abreu FV, Mostardinha P. Maximal frustration as an immunological principle. J R Soc Interface (2009) 6(32):321–34 10.1098/rsif.2008.0280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Leon K, Faro J, Lage A, Carneiro J. Inverse correlation between the incidences of autoimmune disease and infection predicted by a model of T cell mediated tolerance. J Autoimmun (2004) 22(1):31–42 Epub 2004/01/08 10.1016/j.jaut.2003.10.002 [DOI] [PubMed] [Google Scholar]
- 113.Martinez-Pasamar S, Abad E, Moreno B, Velez de Mendizabal N, Martinez-Forero I, Garcia-Ojalvo J, et al. Dynamic cross-regulation of antigen-specific effector and regulatory T cell subpopulations and microglia in brain autoimmunity. BMC Syst Biol (2013) 7:34. 10.1186/1752-0509-7-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Velez de Mendizabal N, Carneiro J, Sole RV, Goni J, Bragard J, Martinez-Forero I, et al. Modeling the effector – regulatory T cell cross-regulation reveals the intrinsic character of relapses in Multiple Sclerosis. BMC Syst Biol (2011) 5:114. 10.1186/1752-0509-5-114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lo WC, Arsenescu RI, Friedman A. Mathematical Model of the Roles of T Cells in Inflammatory Bowel Disease. Bull Math Biol (2013) 75(9):1417–33 10.1007/s11538-013-9853-2 [DOI] [PubMed] [Google Scholar]
- 116.Gross F, Metzner G, Behn U. Mathematical modeling of allergy and specific immunotherapy: Th1-Th2-Treg interactions. J Theor Biol (2011) 269(1):70–8 10.1016/j.jtbi.2010.10.013 [DOI] [PubMed] [Google Scholar]
- 117.Arazi A, Sharabi A, Zinger H, Mozes E, Neumann AU. In vivo dynamical interactions between CD4 Tregs, CD8 Tregs and CD4+ CD25− cells in mice. PLoS One (2009) 4(12):e8447. 10.1371/journal.pone.0008447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bewick S, Yang R, Zhang M. The danger is growing! A new paradigm for immune system activation and peripheral tolerance. PLoS One (2009) 4(12):e8112. 10.1371/journal.pone.0008112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Simonov M, Rawlings RA, Comment N, Reed SE, Shi X, Nelson PW. Modeling adaptive regulatory T-cell dynamics during early HIV infection. PLoS One (2012) 7(4):e33924. 10.1371/journal.pone.0033924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Leon K, Garcia K, Carneiro J, Lage A. How regulatory CD25+CD4+ T cells impinge on tumor immunobiology: the differential response of tumors to therapies. J Immunol (2007) 179(9):5659–68 [DOI] [PubMed] [Google Scholar]
- 121.Leon K, Garcia K, Carneiro J, Lage A. How regulatory CD25(+)CD4(+) T cells impinge on tumor immunobiology? On the existence of two alternative dynamical classes of tumors. J Theor Biol (2007) 247(1):122–37 10.1016/j.jtbi.2007.01.029 [DOI] [PubMed] [Google Scholar]
- 122.Baltcheva I, Codarri L, Pantaleo G, Le Boudec JY. Lifelong dynamics of human CD4+CD25+ regulatory T cells: insights from in vivo data and mathematical modeling. J Theor Biol (2010) 266(2):307–22 10.1016/j.jtbi.2010.06.024 [DOI] [PubMed] [Google Scholar]
- 123.Agua-Doce A, Graca L. Regulatory T cells and the control of the allergic response. J Allergy (2012) 2012:948901 Epub 2012/10/12 10.1155/2012/948901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gratz IK, Rosenblum MD, Abbas AK. The life of regulatory T cells. Ann N Y Acad Sci (2013) 1283:8–12 10.1111/nyas.12011 [DOI] [PubMed] [Google Scholar]
- 125.Perelson AS. Modelling viral and immune system dynamics. Nat Rev Immunol (2002) 2(1):28–36 10.1038/nri700 [DOI] [PubMed] [Google Scholar]