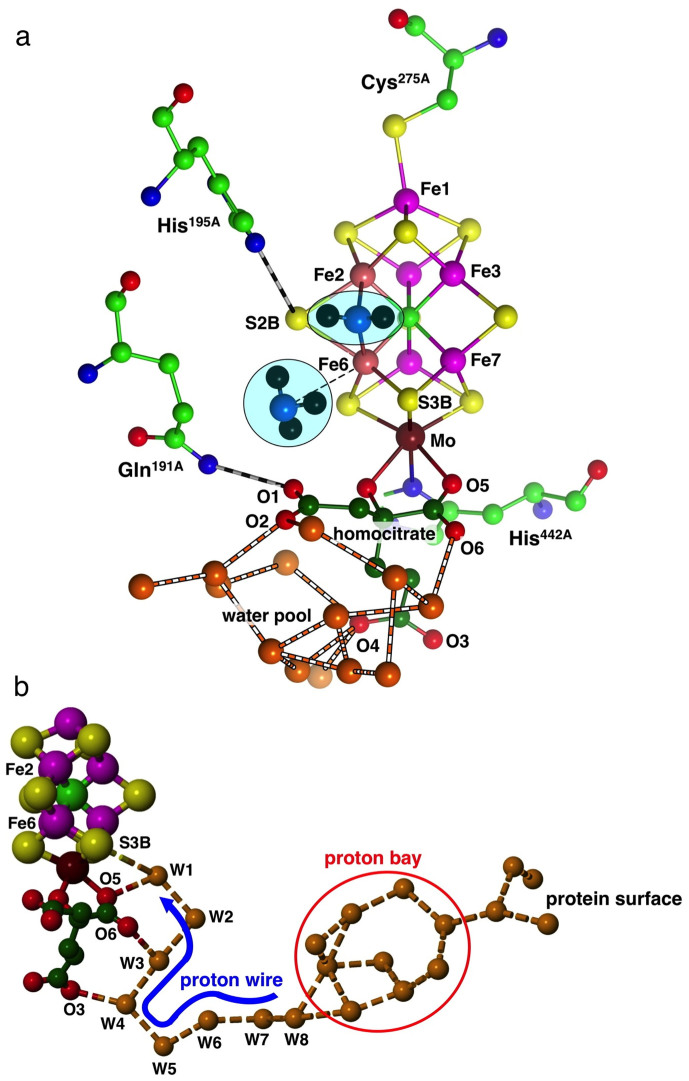
Figure 1.
(a) The structure of FeMo-co including homocitrate (carbon dark green) and ligating residues α-275Cys at Fe1 and α-442His at Mo: atom labels are for the Azotobacter vinelandii protein in PDB 3U7Q. Also shown are key hydrogen bonded residues Gln191A and His195A, and water molecules (orange) of the water pool around homocitrate, The first product NH3 molecule is dissociating from Fe6, the second is to be formed from NH2 bridging Fe2 and Fe6 (in this and some following figures Fe2 and Fe6 are coloured differently). (b) The chain of hydrogen bonded water molecules from the protein surface to S3B of FeMo-co. The proton wire section W8 to W1 is fully conserved, while the branched proton bay section and the path to the surface are variable.