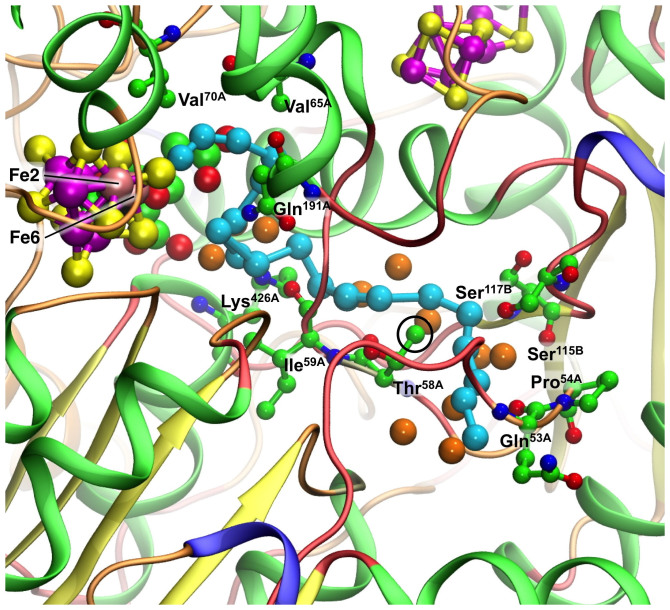
Figure 7. The sequence of modeled positions for NH3 from the reaction face of FeMo-co towards the protein surface.
Cyan spheres are the locations of the N atom: H atoms are not shown (but are included in Supplementary Figs. S2, S3). Orange spheres are water. Key interacting amino acids are marked and labeled. The side chain CH3 of Thr58A (circled) is significant, because the lone pairs of NH3 looping around it are 2.6–2.9Å distant, with the potential for formation of weak C-H → NH3 hydrogen bonds. All protein chains are included and are coloured by structure (α-helix green, extended-β yellow, turn orange, coil red), revealing the minimal secondary structure around most of the proposed NH3 pathway.