Abstract
Pulsed electric field (PEF)-resistant and PEF-sensitive Listeria monocytogenes strains were sublethally treated with electric pulses at 15 kV/cm for 29 μs and held at 25°C for 5 to 30 min prior to protein extraction. The levels of the molecular chaperones GroEL, GroES, and DnaJ were determined by immunoblotting. After 10 to 20 min after sublethal PEF treatment, a transient decrease in molecular chaperone expression was observed in the PEF-sensitive strain (Scott A). The levels of GroEL and DnaJ increased back to the basal expression level within 30 min. A substantial decrease in GroES expression persisted for at least 30 min after PEF treatment. Chaperone expression was suppressed after PEF treatment to a smaller extent in the PEF-resistant (OSY-8578) than in the PEF-sensitive strain, and no clear expression pattern was identified in OSY-8578. Inactivation of Scott A and OSY-8578 in phosphate buffer was compared when lethal PEF (27.5 kV/cm, 144 μs) and heat (55°C, 10 min) were applied in sequence. When PEF and heat treatments were applied separately, the populations of L. monocytogenes Scott A and OSY-8578 decreased 0.5 to 0.6 log CFU/ml. Cells treated first with PEF and incubated at 25°C for 10 min showed substantial sensitivity to subsequent heat treatment; the decrease in counts for Scott A and OSY-8578 was 6.1 and 2.8 log CFU/ml, respectively. The sequence and time lapse between the two treatments were crucial for achieving high inactivation rates. It is concluded that PEF sensitized L. monocytogenes to heat and that maximum heat sensitization occurred when chaperone expression was at a minimum level.
High-intensity electric pulses cause cell electroporation and therefore have useful applications in food preservation, biotechnology, and medicine. These pulses are used to inactivate microorganisms (8), extract cell metabolites (12), manipulate intracellular organelles and macromolecules (6), deliver drugs into tissues (7, 18, 24), and treat patients by gene therapy (24, 33, 39). Pulsed electric field (PEF) is an emerging preservation technology designed to decrease food's microbial load with minimal heating (8). The advantage to food processors is that volatile flavor compounds and thermolabile nutrients are better preserved with PEF than with heat pasteurization (21).
Implementation of emerging technologies, such as PEF, in food preservation depends on successful elimination of pathogens of concern in treated foods. Non-spore-forming food-borne pathogens are commonly targeted by pasteurization, and among these, Listeria monocytogenes has been noted for its high resistance to PEF (1, 9, 20). In a previous study, important variations in PEF sensitivity were found among strains of this pathogen (25). Additionally, L. monocytogenes OSY-8578 was recognized for its high PEF resistance, and it was recommended as a PEF target strain for use in evaluating process efficacy (25). L. monocytogenes Scott A was proposed for use as a PEF-sensitive strain to investigate the range of sensitivity within the genus. Both inducible and constitutive factors were found to contribute to PEF resistance (25). The factor(s) contributing to variations in PEF resistance among these strains is yet to be identified.
Inactivation of microorganisms with PEF is due in part to membrane disruption and leakage of intracellular components during the treatment (39). Electrically induced pores shrink in size in milliseconds but reseal in minutes to hours (36, 44). Conflicting data on detection of PEF-induced cell injury were reported in other studies. According to Russell et al. (34), selective agar plating does not allow the detection of PEF-injured cells. These researchers therefore consider PEF to have an “all or none” effect on treated cells; however, recently cell injury induced by PEF treatment was detected by a vital staining technique (40). It is evident from these studies that the reaction of bacteria to sublethal PEF stress should be investigated further.
Molecular chaperones assist protein folding in the cytoplasm and at the cytoplasm-cell wall interface (27, 41). Treatments that alter the synthesis of molecular chaperones may affect bacterial resistance to processing. Chaperones are among the most highly conserved proteins in nature (35) and are expressed in L. monocytogenes (11, 13, 14). Chaperones such as GroEL, GroES, DnaK, and DnaJ contribute to the intracytoplasmic folding of native proteins and the repair of protein damage (27). These molecular chaperones are known as heat shock proteins because their overexpression in bacteria enhances survival during environmental stresses such as elevated temperature (23, 38). The expression of these chaperones is also higher in the stationary than in the exponential phase of growth (43). Since L. monocytogenes is generally more resistant to PEF in the stationary than in the exponential phase (25), we hypothesized that the ability of this pathogen to maintain molecular chaperone levels contributes to its PEF resistance.
To date, no published information is available on PEF-induced changes in gene and protein expression in bacteria. This research addresses the potential causes of intraspecies L. monocytogenes variability in resistance to PEF. Therefore, the first objective was to compare the expression of major molecular chaperones before and after PEF treatment in PEF-sensitive and PEF-resistant L. monocytogenes strains. Since these chaperones are major heat shock proteins, the second objective was to determine the effect of PEF treatment on the resistance of L. monocytogenes to heat.
MATERIALS AND METHODS
Procedure overview.
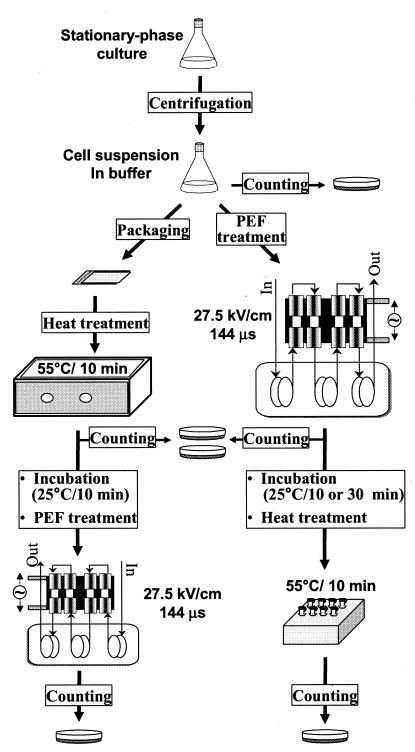
Two separate experiments were performed to assess the effect of PEF on molecular chaperone expression and heat sensitivity of L. monocytogenes. In the first experiment, levels of chaperone expression in untreated and PEF-treated L. monocytogenes were compared as follows. Cells of PEF-sensitive (Scott A) and PEF-resistant (OSY-8578) L. monocytogenes strains were collected at the late exponential phase of growth. Cells were sublethally treated with PEF (15 kV/cm for 29 μs) and incubated for 5 to 30 min at 25°C. Proteins were extracted from untreated and PEF-treated cells, and chaperone levels were determined by immunoblotting. The second experiment examined the relationship between chaperone expression and resistance to the combination of PEF and heat processing (Fig. 1). Cells of Scott A and OSY-8578 cultures were harvested, suspended in phosphate buffer, and treated with lethal levels of PEF (27.5 kV/cm for 144 μs). Cells were also heated at 55°C for 10 min prior to PEF processing or at intervals after the electric treatment. L. monocytogenes survivors were enumerated after PEF, heat, and PEF-heat treatments, and resistance to processing was compared with the expected levels of chaperone expression.
FIG. 1.
Overview of the method for inactivation of L. monocytogenes by sequential PEF and heat treatments.
Microorganisms.
L. monocytogenes Scott A (a clinical isolate) was obtained from the culture collection of the Food Safety Laboratory at Ohio State University. Strain OSY-8578 (isolated from meat) was provided by the Ohio Department of Agriculture (Reynoldsburg, Ohio). Stock cultures were stored at −80°C in tryptose broth (TB; Becton Dickinson, Sparks, Md.) containing 40% (vol/vol) glycerol. L. monocytogenes strains were propagated in TB by incubation at 35°C for 18 h (0.1% inoculum). Cultures were transferred in TB at least twice before use.
Culture preparation and enumeration.
For chaperone analysis, L. monocytogenes was grown in TB at 35°C for ∼6 h, so that a population of ∼1.0 × 108 CFU/ml was obtained. Cultures in TB were sublethally treated with PEF and analyzed for chaperone levels, as indicated below. Treated and untreated cultures were serially diluted in Butterfield phosphate buffer (∼0.3 mM KH2PO4 [pH 7.2]) (Weber Scientific, Hamilton, N.J.) and plated on tryptose agar, and plates were incubated at 35°C for 48 h before enumeration. For PEF inactivation experiments, overnight L. monocytogenes cultures in TB were centrifuged at 6,000 × g and 4°C for 10 min (Sorvall RC-5B; DuPont, Wilmington, Del.) and suspended in 12.5 mM phosphate buffer (KH2PO4), pH 7.2, to a final bacterial concentration of ∼1.0 × 108 CFU/ml. Cell suspensions were treated with PEF, heat, or PEF-heat combinations, as indicated later, and survivors were enumerated on tryptose agar, with incubation at 35°C for 48 h. The efficacy of the treatment was determined as an inactivation value, which was calculated as follows:
 |
PEF equipment.
Cell suspensions were treated in a laboratory-scale PEF processor (OSU-4C; Ohio State University, Columbus), as previously described by Lado and Yousef (25). The PEF processor consisted of four cofield flow chambers in series. The temperature of the cell suspension was adjusted to 22°C after the second and fourth PEF treatment chambers. Temperature control was accomplished by passing the suspension in coils submerged in a cooling water bath. The temperature at the inlet of the first, outlet of the second, inlet of the third, and outlet of the fourth treatment chambers was recorded with digital thermometers (DTM920;Tektronix, Beaverton, Oreg.). An oscilloscope (TDS 340A; Tektronix) monitored the square wave pulse, input voltage, and current.
PEF treatments.
For chaperone analyses, cell suspensions were treated sublethally with an electric field of 15 kV/cm. Bipolar pulses, 3 μs each, were applied at a frequency of 400 Hz. The cell suspension flow rate was 2 ml/s, and thus the total treatment time was 29 μs. The temperature of the cell suspensions did not exceed 35°C during the sublethal PEF treatment. Treated cell suspensions were cooled rapidly, held at 25°C, and analyzed for molecular chaperones after 5, 10, 15, 20, and 30 min of PEF treatment. The cell suspension serving as a control was processed similarly but not treated with electric pulses. For inactivation experiments, cell suspensions were treated with an electric field of 27.5 kV/cm. Bipolar pulses, 3 μs each, were applied at a frequency of 1,000 Hz. The cell suspensions flow rate was adjusted to 1 ml/s to achieve a total treatment time of 144 μs. The temperature of the cell suspensions did not exceed 55°C momentarily during the lethal PEF treatment, and samples were cooled rapidly thereafter. Treated cell suspensions were held at 25°C and analyzed for survivors immediately or 10 or 30 min after PEF treatment. Samples of PEF-treated cell suspensions held at 25°C for 10 and 30 min were heat treated before cells were counted. Each treatment was done at least in triplicate.
Protein purification and molecular chaperone characterization.
Untreated and PEF-treated L. monocytogenes cultures (∼750 ml each) in the late exponential phase of growth were harvested by centrifugation for 15 min at 5,500 × g and 4°C. Cell pellets were suspended in a 200-μl mixture containing 50 mM Tris (pH 7.0), 50 mM EDTA, and 1 mM phenylmethylsulfonyl fluoride. Cells were sonicated five times for 30 s each (pulsed mode; ultrasonic processor XL; Heat Systems, Farmingdale, N.Y.), with 2 min of cooling on ice between bursts. Lysed cells were centrifuged for 20 min at 12,000 × g and 4°C (Fisher Scientific, Pittsburgh, Pa.), and supernatant fluids were collected. The total protein concentration in lysates was determined by the bicinchoninic acid method (Pierce Chemical, Rockford, Ill.). Aliquots containing total protein were mixed with loading buffer (100 mM Tris-HCl [pH 6.8], 5% β-mercaptoethanol, 2% sodium dodecyl sulfate [SDS], 25% glycerol, 0.1% bromophenol blue) at a 1:0.75 ratio (vol/vol). Samples were boiled for 5 min and stored at −20°C until analyzed.
Sample volumes corresponding to 20 μg of total protein (for each sample) were separated by SDS-polyacrylamide gel electrophoresis (PAGE) on 10% polyacrylamide-SDS gels when assessing GroEL and DnaJ levels, while 15% polyacrylamide-SDS gels were used to probe for GroES. Separated protein bands were transferred onto nitrocellulose membranes (Bio-Rad, Hercules, Calif.) and blocked for 1 h with 5% nonfat dry milk in 1× Tris-buffered saline (Bio-Rad) containing 0.1% (vol/vol) Tween 20 (TTBS). Blocked membranes were incubated overnight with primary antibodies (anti-GroEL, 1:2,000; anti-GroES, 1:500; or anti-DnaJ, 1:1,500) at 4°C with gentle shaking. All primary antibodies were obtained from Stressgen Biotechnologies, Victoria, Canada. Membranes were then washed four times (10 min each) in TTBS at 22°C, incubated with 0.5 μl of enzyme-conjugated anti-rabbit immunoglobulin G (horseradish peroxidase conjugate; Cell Signaling Technology, Beverly, Mass.) in 15 ml of TTBS, and washed again in TTBS as indicated above. The membranes were then incubated with a chemiluminescent substrate (Supersignal West Pico kit; Pierce Chemical) according to the manufacturer's instructions and exposed to autoradiography film (X-Omat AR film; Kodak, Rochester, N.Y.). Expression of the three molecular chaperones was quantified with imaging software (Scion Corporation, Frederick, Md.). The band intensity of each PEF-treated sample was compared to that of its corresponding control (untreated sample). PEF-induced changes in molecular chaperone expression were expressed as a percent of the values in untreated controls.
Thermal treatment.
For inactivation experiments (Fig. 1), cell suspensions were heated at 55°C for 10 min and then rapidly cooled to ∼4°C. Heat treatments were done in a water bath (Precision Scientific, Winchester, Va.) or a thermocycler (GeneAmp; Perkin-Elmer, Norwalk, Conn.). The choice of heating device depended on the volume of the cell suspensions. When L. monocytogenes was heated prior to PEF treatment, a large volume of cell suspension was needed for PEF processing. In this case, 150 ml of cell suspension were transferred aseptically to a sterile stomacher bag (∼177 by 250 by 0.35 mm) and vacuum sealed. The sealed bag was immersed in a 55°C water bath and heated for 10 min. To ensure efficient thermal transfer during heating, the bag was held flat so that the cell suspension remained thin. Heat-treated cell suspensions were transferred to an ice-water bath and held for 2 min. When L. monocytogenes was subjected to PEF prior to heating, the PEF-treated cell suspension was held at 25°C and sampled after 10 and 30 min. Aliquots (100 μl each) of the cell suspension were dispensed into 200-μl thin-walled tubes (PCR tubes; Bio-Rad) and heat treated in the thermocycler. Each treatment was done at least in triplicate.
Data analysis for inactivation study.
Counts of L. monocytogenes Scott A and OSY-8578 after PEF, heat, and PEF-heat treatments were compared by the general linear model procedure (SAS, Cary, N.C.), with a priori contrasts and the following statistical model: Count (log CFU per milliliter) = μ + Si + Tj + STij + ɛijk, where Si is the fixed effect of strain (i = 1 or 2) and Tj is the fixed effect of treatment (j = 1,…, 6), where 1 is the control, 2 is PEF, 3 is PEF plus 10 min of incubation plus heat, 4 is PEF plus 30 min of incubation plus heat, 5 is heat, and 6 is heat plus 10 min of incubation plus PEF; STij is the fixed effect of the ith strain by the jth treatment; and ɛijk is the error term, assuming that ɛijk ∼ N(0, σe2). Error variance was considered homogenous. Main effects and interaction were tested with an F test based on a type III sum of squares. When significant, least squares means were compared by Fisher's protected least squares difference (37).
Additionally, a priori contrasts were set before the experiment took place. The P values and estimates of the following contrasts were determined to compare the effect of treatments on L. monocytogenes (regardless of the strain): PEF (T1 versus T2), heat (T1 versus T5), PEF compared to heat (T2 versus T5), single treatment compared to treatment combinations (T3 + T6 versus T2 + T5), PEF treatment compared to the combination of PEF and heat treatments (T2 versus T3 + T4), short delay compared to long delay between PEF and heat treatment (T3 versus T4), and sequence effect of PEF and heat treatments (T3 versus T6). The P values and estimates of the following contrasts were determined to compare sensitivity to treatments: PEF (S1T2 versus S2T2), PEF plus 10 min of incubation plus heat (S1T3 versus S2T3), PEF plus 30 min of incubation plus heat (S1T4 versus S2T4), heat (S1T5 versus S2T5), and heat plus 10 min of incubation plus PEF (S1T6 versus S2T6).
RESULTS
Molecular chaperone expression analysis.
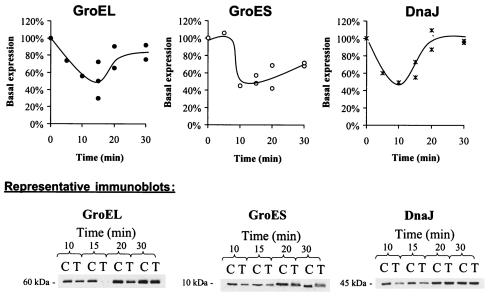
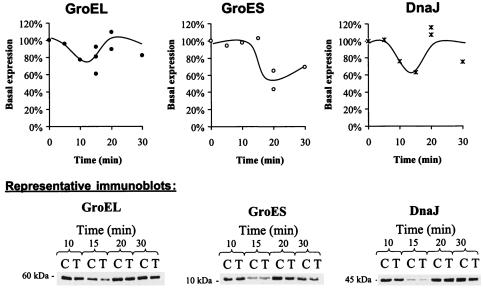
The average basal expression of GroEL in L. monocytogenes during the late exponential phase (∼108 CFU/ml) varied between strains; basal expression for OSY-8578 was only 64% of that for Scott A. Cultures of Scott A and OSY-8578 were sublethally treated with 15 kV/cm for 29 μs to determine within-strain differential expression of chaperones in response to PEF. This treatment inactivated <0.3 log CFU/ml (data not shown). Expression of GroEL, GroES, and DnaJ within 5 to 30 min following PEF treatment was determined (Fig. 2 and 3). The molecular chaperones GroEL, GroES, and DnaJ extracted from L. monocytogenes produced bands corresponding to molecular masses of approximately 60, 10, and 45 kDa, respectively. The experimental conditions used in this study did not allow detection of DnaK. The three chaperones (GroEL, GroES, and DnaJ) exhibited a similar expression pattern in L. monocytogenes Scott A (Fig. 2). Within 15 min post-PEF treatment, 40 to 55% of basal expression was observed for these three chaperones. Thereafter, expression progressively restored the chaperones to their basal levels. Restoration of basal expression levels tended to be slower for GroES than for GroEL and DnaJ; expression of GroES, GroEL, and DnaJ after 30 min of PEF treatment was at ∼70, 83, and 96% of basal expression, respectively. Expression of GroEL and DnaJ by L. monocytogenes OSY-8578 was not altered consistently in response to PEF treatment (Fig. 3). GroES expression in this strain had a pattern similar to that appearing in Scott A. The lowest expression level for GroES was ∼45% and 54% of the basal level in Scott A and OSY-8578, respectively.
FIG. 2.
Expression levels of GroEL, GroES, and DnaJ in L. monocytogenes Scott A after sublethal treatment with PEF at 15 kV/cm for 29 μs and incubation at 25°C for 5 to 30 min. Symbols: •, GroEL; ○, GroES; ×, DnaJ. In the representative immunoblots, C represents the control culture and T represents the PEF-treated samples.
FIG. 3.
Expression levels of GroEL, GroES, and DnaJ in L. monocytogenes OSY-8578 after sublethal treatment with PEF at 15 kV/cm for 29 μs and incubation at 25°C for 5 to 30 min. Symbols: •, GroEL; ○, GroES; ×, DnaJ. In the representative immunoblots, C represents the control culture and T represents the PEF-treated samples.
Sequential PEF and heat challenge.
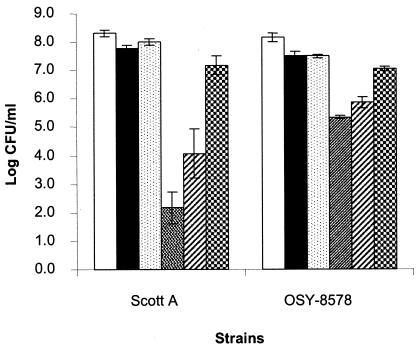
L. monocytogenes, suspended in 12.5 mM phosphate buffer was treated sequentially with mild PEF (27.5 kV for 144 μs) and heat (55°C for 10 min), as illustrated in Fig. 1, and synergy between these treatments was determined. PEF or heat treatment caused a minor yet significant (P < 0.01) decrease in viable count (0.5 to 0.6 log CFU/ml) (Fig. 4). No significant difference in counts (P > 0.05) was found between the PEF-treated and heat-treated L. monocytogenes cultures. Treatment combinations resulted in significantly (P < 0.01) lower counts than did the corresponding single treatments. Sequential application of PEF and heat had a strong synergistic bactericidal activity against L. monocytogenes. When the PEF-treated cell suspension was incubated at 25°C for 10 min and then heated at 55°C for 10 min, the populations of L. monocytogenes Scott A and OSY-8578 decreased 6.1 and 2.8 log CFU/ml, respectively. Sensitivity to heat decreased significantly (P < 0.01) when the lag between PEF and heat treatments was increased to 30 min. In the latter case, populations of L. monocytogenes Scott A and OSY-8578 decreased 4.3 and 2.3 log CFU/ml, respectively. On the contrary, when L. monocytogenes was first heated and then incubated at 25°C for 10 min and treated with PEF, only an additive bactericidal effect was observed. L. monocytogenes populations decreased 1.2 log CFU/ml after this treatment combination. When comparing the two strains, Scott A was significantly more sensitive than OSY-8578 to heat (P < 0.05) and PEF followed by heat (P < 0.001). However, inactivation of Scott A was not significantly different (P > 0.05) from that of OSY-8578 when PEF or heat followed by PEF was applied.
FIG. 4.
Viable counts of L. monocytogenes Scott A and OSY-8578 suspended in 12.5 mM phosphate buffer (pH 7.2) and treated with mild PEF (27.5 kV/cm for 144 μs) and heat (55°C for 10 min). From left to right within each group, the bars represent the untreated control, PEF treatment, heat treatment, PEF treatment followed by 10 min of incubation at 25°C prior to heat treatment, PEF treatment followed by 30 min of incubation at 25°C prior to heat treatment, and heat treatment followed by 10 min of incubation at 25°C prior to PEF treatment.
DISCUSSION
The first objective of this study was to determine whether PEF treatment alters the expression of major molecular chaperones in L. monocytogenes. Limited information is available in the published literature on antibodies used against Listeria chaperones (17, 31). We successfully detected L. monocytogenes GroEL, GroES, and DnaJ with commercial antibodies developed for the corresponding Escherichia coli chaperones. L. monocytogenes GroEL (∼60 kDa), GroES (∼10 kDa), and DnaJ (∼45 kDa) had molecular masses comparable to these of E. coli (57, 10, and 41 kDa, respectively) (32). The E. coli DnaK antibody used in this study may not have been specific for Listeria DnaK. Therefore, DnaK could not be detected in this study.
Basal expression of GroEL was higher in the PEF-sensitive (Scott A) than in PEF-resistant (OSY-8578) L. monocytogenes strain. Interestingly, earlier studies (17, 31) reported higher levels of this chaperone in phagocytosis-sensitive than in phagocytosis-resistant L. monocytogenes. PEF treatment decreased the expression of at least three molecular chaperones, GroEL, GroES, and DnaJ. Altered expression of chaperones is commonly observed when exponential-phase cells are exposed to sublethal stress (15). Total PEF treatment time for food processing commonly ranges from microseconds to milliseconds. This short treatment may be sufficient to trigger a stress response, but cells require at least 5 min before altered expression of stress proteins becomes detectable at the mRNA level (11). Consistent with this observation, the level of GroEL expressed by L. monocytogenes was lowest after 10 to 15 min of sublethal PEF treatment (Fig. 2).
Synthesis of GroEL, GroES, DnaJ, and DnaK generally increases upon environmental stress (2, 11, 15). Magnetic fields also induce overproduction of DnaJ and DnaK in E. coli (5). Therefore, a transient decrease in GroEL, GroES, and DnaJ expression levels in response to PEF treatment was unexpected. The pattern of chaperone expression in PEF-treated listeriae, on the other hand, showed analogies with the decreased expression of GroEL and DnaK observed in murine macrophages (17). Complete suppression of GroEL synthesis would lead to cell lysis (30). Kanemori et al. (22) found that downregulation of cellular GroEL and GroES in E. coli to ∼25% of basal levels only slightly reduced the growth rate. This observation implies that chaperones at 25% of basal levels would still be sufficient to support bacterial growth. Similarly, PEF-treated cells that were incubated at 25°C for 15 min and subsequently grown in acidified tryptose broth (pH 5.0) had growth patterns similar to that of the untreated cells (data not shown).
The decrease in chaperone levels may be associated with pore formation in cell membranes upon treatment with PEF. These pores may permit the leakage of cell chaperones. As incubation time increases, pore size diminishes, and only small molecules may leave the treated cell. Consistent with this hypothesis, the level of the smallest chaperone (GroES) 30 min after the PEF treatment was lower than that of GroEL or DnaJ; however, the delayed decrease in chaperone levels after PEF treatment indicated that the size of the electrically induced pores was not sufficient for passive diffusion of GroES (∼10 kDa), DnaJ (∼45 kDa), and GroEL (∼60 kDa). Recent studies suggest that environmental stress may induce the association of GroEL with the cell membrane, and subsequently the chaperone is localized extracellularly without the involvement of cell lysis (15, 16). Chaperone transfer across the membrane may therefore involve a two-step mechanism analogous to plasmid uptake by electroporation; the electric pulse induces anchoring of the chaperones to the membrane, and these proteins then slowly cross the membrane (10).
The time lag between PEF treatment and the maximum decline in chaperone levels (10 to 20 min) seemed to match that required for de novo protein synthesis. Sublethal PEF treatment may have temporarily disrupted transcription. Consequently, the levels of de novo chaperone synthesis diminished 10 to 20 min after the treatment and recovered thereafter. Heat shocking listeriae at 45°C increased GroEL transcription within 5 min. The highest level of the chaperone was attained within 15 min of treatment (11). This indicates that delayed depression of chaperone levels after PEF treatment may be due to a downregulation of chaperone synthesis. The decrease in chaperone levels may also have resulted from an increased rate of destruction of these proteins. The transcription levels of these chaperones after PEF treatment will be compared to the expression of housekeeping genes in future experiments.
The decrease in the molecular chaperones in L. monocytogenes Scott A paralleled the increased sensitization of this pathogen to mild heat. When sufficient time was given for the cells to recover from PEF treatment, chaperone levels increased and so did heat resistance. Landry et al. (26) observed a decay in heat shock protein expression that corresponded to a reduction in thermotolerance. GroEL, GroES, and DnaJ are major heat shock proteins, and they have been studied extensively for their involvement in the refolding of damaged proteins (27, 29). GroEL binds to 10 to 15% of newly synthesized proteins in unstressed cells and up to 30% after mild heat shock (19). Thermal treatments similar to that tested in this study (55°C) do not protect L. monocytogenes against more severe heat treatment (4). Therefore, it is unlikely that chaperones are overexpressed at this treatment temperature. Compared to L. monocytogenes Scott A, OSY-8578 is more PEF resistant when suspended in 0.1% NaCl or acid whey (25). In the current study, PEF treatments were too mild to reveal variations in PEF resistance between L. monocytogenes strains; however, after PEF-treated cells were heated, OSY-8578 was significantly more resistant to the combined treatment than was Scott A. Chaperone levels in the PEF-resistant strain were less altered than they were in the PEF-sensitive strain. This finding may prove the association between PEF resistance and maintenance of high chaperone levels after electroporation.
The PEF and heat processing parameters used in this study were milder than are generally recommended for bacterial decontamination; however, the mild treatments facilitated quantification of cell viability when sequential PEF and heat treatments were applied to the culture. The PEF-sensitive L. monocytogenes Scott A was remarkably more resistant to PEF treatment in 12.5 mM phosphate buffer than was previously found in 17 mM sodium chloride solution (25). These two suspending media have similar conductivities (0.2 S/m) and osmolarities. The relative sensitivity of L. monocytogenes to PEF in the sodium chloride solution may therefore be due to slight electrolysis during the treatment and hence the production of oxidative species such as HOCl. Heat inactivation levels were in agreement with D values reported by Linton et al. (28) and Walsh et al. (42). Sequential applications of PEF followed by heat and heat followed by PEF were not equivalent in lethality. Treatment of L. monocytogenes with PEF followed by heat resulted in a strong synergism between these two inactivation techniques. Post-PEF decreases in chaperone expression and heat resistance suggest that chaperones play an important role in the resistance of L. monocytogenes to mild heat treatment. Heat increases membrane permeability, denatures cell proteins, causes single-strand breaks in DNA, and degrades RNA (3).
In conclusion, PEF treatment altered the expression of the molecular chaperones GroEL, GroES, and DnaJ. Cells with altered chaperone expression became sensitive to mild heat treatment. Therefore, PEF treatment facilitated the bactericidal action of heat in a time-dependent manner. These findings may assist food processors in optimizing PEF treatments and enhance treatment efficacy against L. monocytogenes.
Acknowledgments
The Center for Advanced Food Processing and Packaging Studies supported this research.
We thank Normand St-Pierre and Xing-Ya Wang for valuable technical assistance.
REFERENCES
- 1.Aronsson, K., M. Lindgren, B. R. Johansson, and U. Ronner. 2001. Influence of microorganisms using pulsed electric fields: the influence of process parameters on Escherichia coli, Listeria innocua, Leuconostoc mesenteroides and Saccharomyces cerevisiae. Innov. Food Sci. Emerg. Technol. 2:41-54. [Google Scholar]
- 2.Arsene, F., T. Tomoyasu, and B. Bukau. 2000. The heat shock response of Escherichia coli. Int. J. Food Microbiol. 55:3-9. [DOI] [PubMed] [Google Scholar]
- 3.Bunduki, M. M. C., K. J. Flanders, and C. W. Donnelly. 1995. Metabolic and structural sites of damage in heat- and sanitizer-injured populations of Listeria monocytogenes. J. Food Prot. 58:410-415. [DOI] [PubMed] [Google Scholar]
- 4.Bunning, V. K., R. G. Crawford, J. T. Tierney, and J. T. Peeler. 1990. Thermotolerance of Listeria monocytogenes and Salmonella typhimurium after sublethal heat shock. Appl. Environ. Microbiol. 56:3216-3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chow, K., and W. L. Tung. 2000. Magnetic field exposure enhances DNA repair through the induction of DnaK/J. synthesis. FEBS Lett. 478:133-136. [DOI] [PubMed] [Google Scholar]
- 6.Deng, J., K. H. Schoenbach, E. S. Buescher, P. S. Hair, P. M. Fox, and S. J. Beebe. 2003. The effects of intense submicrosecond electrical pulses on cells. Biophys. J. 84:2709-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dujardin, N., E. Staes, Y. Kalia, P. Clarys, R. Guy, and V. Preat. 2002. In vivo assessment of skin electroporation using square wave pulses. J. Control Release 79:219-227. [DOI] [PubMed] [Google Scholar]
- 8.Dunn, J. 2001. Pulsed electric field processing: an overview, p. 1-30. In G. V. Barbosa-Canovas and Q. H. Zhang (ed.), Pulsed electric fields in food processing: fundamental aspects and applications. Technomic Publishing Co., Lancaster, Pa.
- 9.Dutreux, N., S. Notermans, T. Wijtzes, M. M. Gongora-Nieto, G. V. Barbosa-Canovas, and B. G. Swanson. 2000. Pulsed electric fields inactivation of attached and free-living Escherichia coli and Listeria innocua under several conditions. Int. J. Food Microbiol. 54:91-98. [DOI] [PubMed] [Google Scholar]
- 10.Eynard, N., S. Sixou, N. Duran, and J. Teissie. 1992. Fast kinetics studies of Escherichia coli electrotransformation. Eur. J. Biochem. 209:431-436. [DOI] [PubMed] [Google Scholar]
- 11.Gahan, C. G., J. O'Mahony, and C. Hill. 2001. Characterization of the groESL operon in Listeria monocytogenes: utilization of two reporter systems (gfp and hly) for evaluating in vivo expression. Infect. Immun. 69:3924-3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ganeva, V., B. Galutzov, and J. Teissie. 2003. High yield electroextraction of proteins from yeast by a flow process. Anal. Biochem. 315:77-84. [DOI] [PubMed] [Google Scholar]
- 13.Hanawa, T., M. Fukuda, H. Kawakami, H. Hirano, S. Kamiya, and T. Yamamoto. 1999. The Listeria monocytogenes DnaK chaperone is required for stress tolerance and efficient phagocytosis with macrophages. Cell Stress Chaperones 4:118-128. [PMC free article] [PubMed] [Google Scholar]
- 14.Hanawa, T., M. Kai, S. Kamiya, and T. Yamamoto. 2000. Cloning, sequencing, and transcriptional analysis of the dnaK heat shock operon of Listeria monocytogenes. Cell Stress Chaperones 5:21-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hennequin, C., A. Collignon, and T. Karjalainen. 2001. Analysis of expression of GroEL (Hsp60) of Clostridium difficile in response to stress. Microb. Pathog. 31:255-260. [DOI] [PubMed] [Google Scholar]
- 16.Hennequin, C., F. Porcheray, A. Waligora-Dupriet, A. Collignon, M. Barc, P. Bourlioux, and T. Karjalainen. 2001. GroEL (Hsp60) of Clostridium difficile is involved in cell adherence. Microbiology 147:87-96. [DOI] [PubMed] [Google Scholar]
- 17.Hevin, B., M. Morange, and R. M. Fauve. 1993. Absence of an early detectable increase in heat-shock protein synthesis by Listeria monocytogenes within mouse mononuclear phagocytes. Res. Immunol. 144:679-689. [DOI] [PubMed] [Google Scholar]
- 18.Hofmann, G. A., S. B. Dev, G. S. Nanda, and D. Rabussay. 1999. Electroporation therapy of solid tumors. Crit. Rev. Ther. Drug Carrier Syst. 16:523-569. [PubMed] [Google Scholar]
- 19.Houry, W. A., D. Frishman, C. Eckerskorn, F. Lottspeich, and F. U. Hartl. 1999. Identification of in vivo substrates of the chaperonin GroEL. Nature 402:147-154. [DOI] [PubMed] [Google Scholar]
- 20.Hulsheger, H., J. Potel, and E. G. Niemann. 1983. Electric field effects on bacteria and yeast cells. Radiat. Environ. Biophys. 22:149-162. [DOI] [PubMed] [Google Scholar]
- 21.Jia, M., Q. H. Zhang, and D. B. Min. 1999. Pulsed electric field processing effects on flavor compounds and microorganisms of orange juice. Food Chem. 65:445-451. [Google Scholar]
- 22.Kanemori, M., H. Mori, and T. Yura. 1994. Effects of reduced levels of GroE chaperones on protein metabolism: enhanced synthesis of heat shock proteins during steady-state growth of Escherichia coli. J. Bacteriol. 176:4235-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kilstrup, M., S. Jacobsen, K. Hammer, and F. K. Vogensen. 1997. Induction of heat shock proteins DnaK, GroEL, and GroES by salt stress in Lactococcus lactis. Appl. Environ. Microbiol. 63:1826-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krassowska, W., G. S. Nanda, M. B. Austin, S. B. Dev, and D. P. Rabussay. 2003. Viability of cancer cells exposed to pulsed electric fields: the role of pulse charge. Ann. Biomed. Eng. 31:80-90. [DOI] [PubMed] [Google Scholar]
- 25.Lado, B. H., and A. E. Yousef. 2003. Selection and identification of a Listeria monocytogenes target strain for pulsed electric field process optimization. Appl. Environ. Microbiol. 69:2223-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landry, J., D. Bernier, P. Chretien, L. M. Nicole, R. M. Tanguay, and N. Marceau. 1982. Synthesis and degradation of heat shock proteins during development and decay of thermotolerance. Cancer Res. 42:2457-2461. [PubMed] [Google Scholar]
- 27.Langer, T., C. Lu, H. Echols, J. Flanagan, M. K. Hayer, and F. U. Hartl. 1992. Successive action of DnaK, DnaJ and GroEL along the pathway of chaperone-mediated protein folding. Nature 356:683-689. [DOI] [PubMed] [Google Scholar]
- 28.Linton, R. H., M. D. Pierson, and J. R. Bishop. 1990. Increase in heat resistance of Listeria monocytogenes Scott A by sublethal heat shock. J. Food Prot. 53:924-927. [DOI] [PubMed] [Google Scholar]
- 29.Lund, P. A. 2001. Microbial molecular chaperones. Adv. Microb. Physiol. 44:93-140. [DOI] [PubMed] [Google Scholar]
- 30.McLennan, N., and M. Masters. 1998. GroE is vital for cell-wall synthesis. Nature 392:139. [DOI] [PubMed] [Google Scholar]
- 31.Morange, M., B. Hevin, and R. M. Fauve. 1993. Differential heat-shock protein synthesis and response to stress in three avirulent and virulent Listeria species. Res. Immunol. 144:667-677. [DOI] [PubMed] [Google Scholar]
- 32.Nam, S. H., and M. K. Walsh. 2002. Affinity purification and characterization of the Escherichia coli molecular chaperones. Protein Expr. Purif. 24:282-291. [DOI] [PubMed] [Google Scholar]
- 33.Oshima, Y., T. Sakamoto, I. Yamanaka, T. Nishi, T. Ishibashi, and H. Inomata. 1998. Targeted gene transfer to corneal endothelium in vivo by electric pulse. Gene Ther. 5:1347-1354. [DOI] [PubMed] [Google Scholar]
- 34.Russell, N. J., M. Colley, R. K. Simpson, A. J. Trivett, and R. I. Evans. 2000. Mechanism of action of pulsed high electric field (PHEF) on the membranes of food-poisoning bacteria is an 'all-or-nothing' effect. Int. J. Food Microbiol. 55:133-136. [DOI] [PubMed] [Google Scholar]
- 35.Segal, R., and E. Z. Ron. 1996. Regulation and organization of the groE and dnaK operons in eubacteria. FEMS Microbiol. Lett. 138:1-10. [DOI] [PubMed] [Google Scholar]
- 36.Serpersu, E. H., K. Kinosita, Jr., and T. Y. Tsong. 1985. Reversible and irreversible modification of erythrocyte membrane permeability by electric field. Biochim. Biophys. Acta 812:779-785. [DOI] [PubMed] [Google Scholar]
- 37.Snedecor, G. W., and W. G. Cochran. 1980. Statistical methods. Iowa State University Press, Ames, Iowa.
- 38.Tomoyasu, T., T. Ogura, T. Tatsuta, and B. Bukau. 1998. Levels of DnaK and DnaJ provide tight control of heat shock gene expression and protein repair in Escherichia coli. Mol. Microbiol. 30:567-581. [DOI] [PubMed] [Google Scholar]
- 39.Tsong, T. Y. 1991. Electroporation of cell membranes. Biophys. J. 60:297-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Unal, R., A. E. Yousef, and P. C. Dunne. 2002. Spectrophotometric assessment of bacterial cell membrane damage by pulsed electric field. Innov. Food Sci. Emerg. Technol. 3:247-254. [Google Scholar]
- 41.Wahlstrom, E., M. Vitikainen, V. P. Kontinen, and M. Sarvas. 2003. The extracytoplasmic folding factor PrsA is required for protein secretion only in the presence of the cell wall in Bacillus subtilis. Microbiology 149:569-577. [DOI] [PubMed] [Google Scholar]
- 42.Walsh, D., J. J. Sheridan, G. Duffy, I. S. Blair, D. A. McDowell, and D. Harrington. 2001. Thermal resistance of wild-type and antibiotic-resistant Listeria monocytogenes in meat and potato substrates. J. Appl. Microbiol. 90:555-560. [DOI] [PubMed] [Google Scholar]
- 43.Wise, A. A., and C. W. Price. 1995. Four additional genes in the sigB operon of Bacillus subtilis that control activity of the general stress factor sigma B in response to environmental signals. J. Bacteriol. 177:123-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xie, T. D., L. Sun, and T. Y. Tsong. 1990. Study of mechanisms of electric field-induced DNA transfection. I. DNA entry by surface binding and diffusion through membrane pores. Biophys. J. 58:13-19. [DOI] [PMC free article] [PubMed] [Google Scholar]