Abstract
A high-performance liquid chromatography method was developed for the estimation of ferulic acid from asafoetida and a polyherbal preparation. The separation was carried out on HiQSil ODS C-18 column with a mobile phase of acetonitrile: 10% acetic acid (20:80 v/v). The developed method was validated as per International Conference of Harmonization guidelines for various parameters such as accuracy, precision, linearity, limit of detection, limit of quantification and specificity; and found to be reliable. Linear regression analysis showed a good corelation between peak area and concentration with a corelation coefficient r2=0.996 in the range 200-7000 ng/ml. The developed method can be utilized for standardization of herbal formulation comprising asafoetida.
Keywords: High-performance liquid chromatograhy, asafoetida, ferulic acid, polyherbal formulations.
Ferula asafoetida belonging to family Umbelliferae is a tall perennial plant which grows upto 2 m and requires dry moist soil. The dried latex, an oleo-gum-resin, known as asafoetida is obtained by making deep incision in the roots and rhizomes; and is widely preferred for culinary purpose as well as for medicinal use[1]. Asafoetida chiefly comprises resin (40-65%), gum (20-25%), and volatile oil (4-20%). The asafoetida comprises a number of sesquiterpenes of which assaresinotannol is the chief sesquiterpene present in either free form or in combined form with ferulic acid or galbanic acid, also free ferulic acid is reported to be present in asafoetida[2]. Studies have revealed that asafoetida exhibits numerous pharmacological activities such as the antispasmodic[3,4], antifungal[5], antioxidant[6,7], antidiabetic[8], antimicrobial[9], antiulcer[10], antihemolytic[7], chemopreventive[11,12] and antiviral[13].
Ferulic acid is a phenolic acid present in asafoetida which exhibits numerous activities such as an anticancer, antioxidant and others[14,15]. Therefore, a reverse phase high-performance liquid chromatography (HPLC) method has been developed to quantitatively estimate the ferulic acid content in asafoetida. The developed method can be employed as a quality control tool for numerous herbal preparations containing asafoetida.
The solvents methanol, acetonitrile, and acetic acid used for study were all of HPLC grade and obtained from S. D. Fine Chemicals, Mumbai. Ferulic acid was purchased from P. C. Chem, Mumbai. The drug asafoetida was purchased from Yucca Enterprises, Mumbai. The marketed polyherbal preparation hinguvachadi tablet was purchased from Kotaikkal Arya Vaidya Sala, Mumbai.
HPLC studies were carried out on Agilent 1200 series operated by software EZ-Chrome Elite and separations were achieved on a reversed–phase HiQSil C-18 column with a dimension of 250×4.6 mm and a particle size of 5 μ. The mobile phase used for analysis comprised acetonitrile and 10% acetic acid (20:80 v/v). The pH of the mobile phase was found to be 2.25 and the flow rate was kept at 1.0 ml/min. The analysis was carried out at the column oven temperature of 30° and 20 μl of samples were injected into the column. The wavelength used for detection purpose was 319 nm.
The standard solution of ferulic acid were prepared at concentrations of 200, 600, 1800, 2600, 4000, 5000, 6000, and 7000 ng/ml using methanol as a solvent and were injected in triplicate, the detector response were measured for constructing the calibration curve. Accurately weighed 25 g of asafoetida powder was extracted with 25 ml methanol. The extraction cycle was repeated for three times. The filtrates were combined, concentrated and dried to get an extract, which was used for further analysis. The yield of the extract was 5% w/w. A 0.04% w/v methanol solution of extract was prepared. The extract solution was filtered through a 0.45 μ filter and assayed in triplicate, the peak area corresponding to ferulic acid was compared with the calibration curve and the amount of ferulic acid was determined.
The developed method was validated for parameters such as linearity, accuracy, precision, limit of detection (LOD), limit of quantification (LOQ), and specificity. For accuracy studies about 50, 100, and 150% of standard ferulic acid was added to the prequantified samples and subjected to analysis. The precision of system was determined by measuring the repeatability of six samples injected and measuring their corresponding peak areas. In order to evaluate the intraday precision six samples at three different concentrations were analyzed on the same day and interday precision, was evaluated by analyzing the samples on three different days. The specificity of a compound was determined by analyzing and comparing the Rt of the compound of interest, ferulic acid from the sample to that of the standard.
The marketed preparation Hinguvachadi tablet was analyzed by the aforesaid chromatographic conditions for the presence of ferulic acid in it. Accurately weighed 10 g of the powdered tablet was extracted with methanol similar to asafoetida powder and concentrated to get an extract. A 0.4% w/v methanol solution of extract was prepared and subjected for analysis.
A number of mobile phases were tried in order to get the pH of the mobile phase within the range of 2-2.5 for better separation of phenolic compounds[16]. The mobile phase comprising acetonitrile and 10% acetic acid in the ratio 20:80 was selected. The mobile phase yielded a peak of ferulic acid at Rt of 10.24 (fig. 1).
Fig. 1.
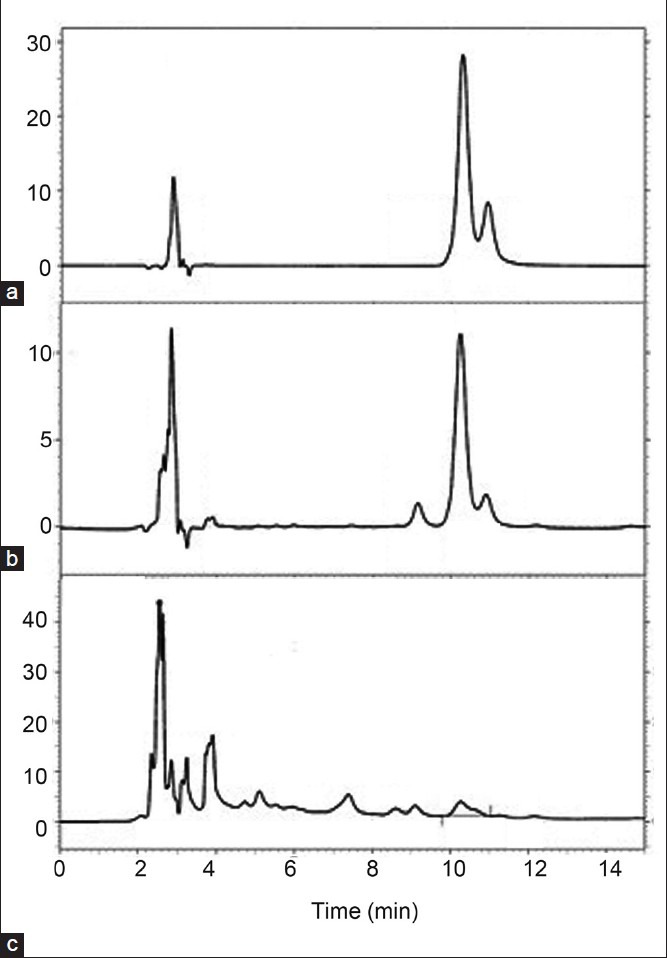
HPLC chromatograms of standard, asafoetida methanol extract, and polyherbal preparation.
Chromatograms showing. (a) ferulic acid (b) methanol extract of asafoetida and (c) polyherbal preparation.
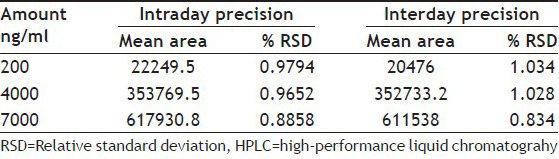
A good linear relationship was obtained when a graph was plotted for concentration v/s ferulic acid peak area with a correlation coefficient r2=0.996 in the concentration range of 200 to 7000 ng/ml. The equation of linear regression equation was y=91.97x+8021. The recovery of ferulic acid was in the range of 103.13-104.14% with % RSD less than 2% (Tables 1 and 2). The LOD and LOQ of the method were found to be 105 and 353 ng/ml, respectively. The % RSD for repeatability of sample application was found to be 0.638% and 0.535%. The % RSD for interday and intraday precision was found out to be less than 2% (Table 3). There were no other interfering spots by other constituents of the extract at the Rt value of the standard ferulic acid which indicated the specificity of the developed method. The concentration of ferulic acid in asafoetida and hinguvachadi tablet was found to be 0.043 and 0.004% w/w.
TABLE 1.
ACCURACY AS RECOVERY
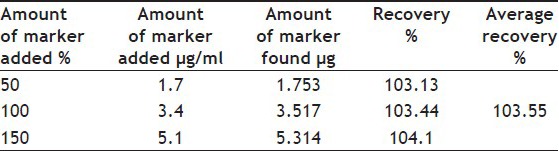
TABLE 2.
STATISTICAL VALIDATION OF RECOVERY DATA

TABLE 3.
INTRADAY AND INTERDAY PRECISION OF HPLC METHOD
The developed HPLC method was found to be sensitive, precise, specific, and reliable for the determination of ferulic acid in asafoetida and can be widely employed as a quality control tool for asafoetida, its extracts and formulations containing asafoetida.
Footnotes
Kareparamban, et al.: Estimation of Ferulic Acid in Asafoetida by HPLC
REFERENCES
- 1.Shah B, Seth A. 1st ed. New York: Elsevier; 2010. Textbook of Pharmacognosy and Phytochemistry; pp. 319–21. [Google Scholar]
- 2.Wallis TE. 5th ed. New York: CBS Publisher; 2004. Textbook of Pharmacognosy; pp. 503–5. [Google Scholar]
- 3.Mohammad F, Freshteh F, Hassanabad ZF. Antispasmodic and hypotensive activity of Ferula asafoetida gum extract. J Ethnopharmacol. 2004;91:321–4. doi: 10.1016/j.jep.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Gholamnezhad Z, Byrami G, Boskabady MH. Possible mechanism of the relaxant effect of asafoetida. Avicenna J Phytomed. 2012;2:10–6. [Google Scholar]
- 5.Houghton PJ, Apendino G, Maxia L. A meroterpenoid NF-ĸβ inhibitor and drimane sesquiterpenoids from asafoetida. J Nat Prod. 2006;69:1101–4. doi: 10.1021/np0600954. [DOI] [PubMed] [Google Scholar]
- 6.Dehpour AA, Ebrahimzadeh MA, Fazel NS. Antioxidant activity of methanolic extract of Ferula assafoetida and its essential oil composition. Grasas Y Aceites. 2009;60:405–12. [Google Scholar]
- 7.Nabavi SM, Ebrahimzadeh MA, Nabavi SE. Antioxidant and antihaemolytic activity of Ferula foetida regel. Eur Rev Med Pharmacol Sci. 2011;15:157–64. [PubMed] [Google Scholar]
- 8.Abu Zaiton AS. Antidiabetic activity of Ferula assafoetida extract in normal and alloxan-induced diabetic rats. Pak J Biol Sci. 2010;13:97–100. doi: 10.3923/pjbs.2010.97.100. [DOI] [PubMed] [Google Scholar]
- 9.Mishra N, Behal KK. Antimicrobial activity of some spices against selected microbes. Int J Pharm Pharm Sci. 2010;2:187–96. [Google Scholar]
- 10.Alqasoumi S, Al-Dosari M, Al-Howiriny T. Gastric antiulcer activity of a pungent spice Ferula assafoetida in rats. Farmacia. 2011;59:750–9. [Google Scholar]
- 11.Mohammad S, Alam A, Sultana S. Asafoetida inhibits early events of carcinogenesis: A chemopreventive study. Life Sci. 2001;68:1913–21. doi: 10.1016/s0024-3205(01)00977-8. [DOI] [PubMed] [Google Scholar]
- 12.Mishra N, Behal KK. Chemopreventive activity of some spices against selected cell line. Der Pharm Sin. 2011;2:31–5. [Google Scholar]
- 13.Lee CL, Chiang CL, Cheng HL. Influenza A (H1N1) and cytotoxic agents from Ferula assafoetida. J Nat Prod. 2009;30:1–5. doi: 10.1021/np900158f. [DOI] [PubMed] [Google Scholar]
- 14.Baskaran N, Manoharan S, Balakrishnan S. Chemopreventive potential of ferulic acid in 7,12-dimethylbenz[a] anthracene-induced mammary carcinogenesisin Sprague-Dawley rats. Eur J Pharmacol. 2010;637:22–9. doi: 10.1016/j.ejphar.2010.03.054. [DOI] [PubMed] [Google Scholar]
- 15.Cheng CY, Su SY, Tang NY. Ferulic acid provides nueroprotection against oxidative stress-related apoptosis after cerebral ischemia/perfusion injury by inhibiting ICAM-1 mRNA expression in rats. Brain Res. 2008;1209:136–50. doi: 10.1016/j.brainres.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 16.Sherma J, Bernard F. 3rd ed. New York: Marcel Dekker; 2003. Handbook of Thin-Layer Chromatography; pp. 52–7. [Google Scholar]


