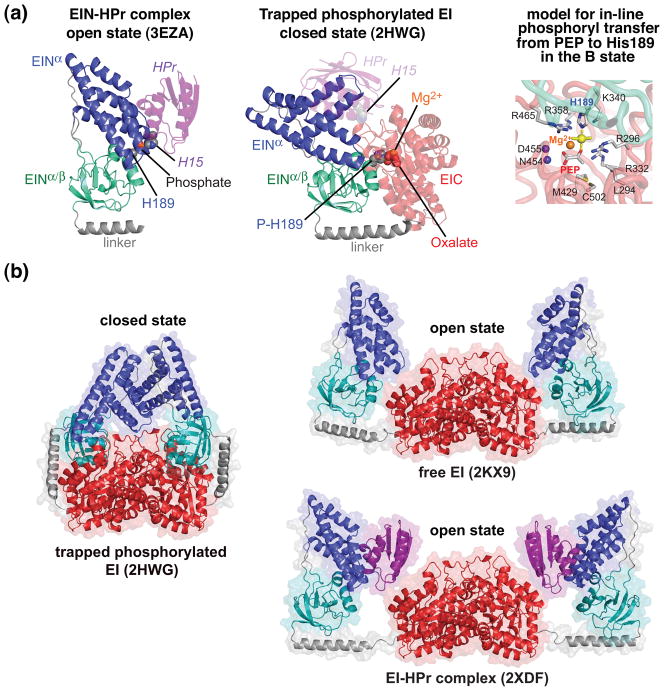
Fig. 4.
Comparison of open and closed states of EI. (a) The open state is found in the isolated EIN domain (left panel) and in intact EI both free and in complex with HPr [30, 39–42]; the closed state (middle panel) is found in the crystal structure of the trapped phosphorylated intermediate of EI [33]. The EINα/β subdomain (cyan) is shown in the same orientation in both panels. Only a single subunit of phosphorylated EI is shown. The color coding is as follows: EINα, blue; EINα/β, cyan; EIC, red; linker connecting EINα/β to EIC, brown; HPr, purple. HPr bound to the EINα subdomain of phosphorylated EI (closed state) in the same orientation as in the EIN-HPr complex (open state) is shown in the middle panel as a transparent purple ribbon to illustrate that the HPr binding site is available in the closed state and there are no clashes between HPr and EIC in this conformation, but that the distance between His15 of HPr and His189 of EINα is much too large (~30 Å) to allow phosphoryl transfer from EIN to HPr to take place in the closed state. The right panel shows a model of in-line phosphoryl transfer from PEP to His189 in the closed state. (b) Comparison of the crystal structure of the trapped phosphorylated intermediate of the EI dimer (closed state) with the solution structures of the free EI dimer and the dimeric EI-HPr complex (open state) determined from combined use of NMR residual dipolar couplings and solution X-ray scattering (SAXS/WAXS). The color coding is the same as in (a).