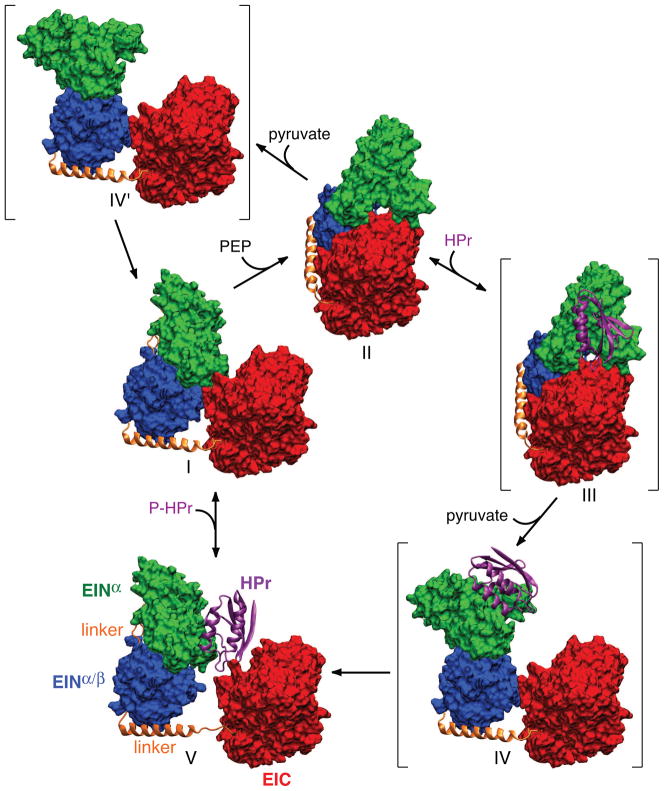
Fig. 5.
Catalytic cycle of EI. Only a single subunit is displayed for clarity with the molecular surfaces of the EINα and EINα/β subdomains of EIN in green and blue, respectively, the molecular surface of EIC domain in red, the linker connecting EINα/β to EIC in a gold ribbon, and HPr in a purple ribbon. Structures I and V correspond to free EI and the EI-HPr complex (open state) solved by NMR and SAXS/WAXS, structure II to the crystal structure of the trapped phosphorylated intermediate (closed state), and structures III, IV and IV′ (shown in brackets) to postulated intermediates. Structure III corresponds to the binding of HPr to the trapped phosphorylated intermediate; structures IV and IV′ correspond to structures in which the orientation of the EINα/β subdomain relative to EIC is the same as that in free EI (I) or the EI-HPr complex (V), while the orientation of the EINα subdomain relative to EINα/β is the same as that in the trapped phosphorylated intermediate (II). The EIC domain (red) is displayed in the same orientation for all structures. Adapted from [40].