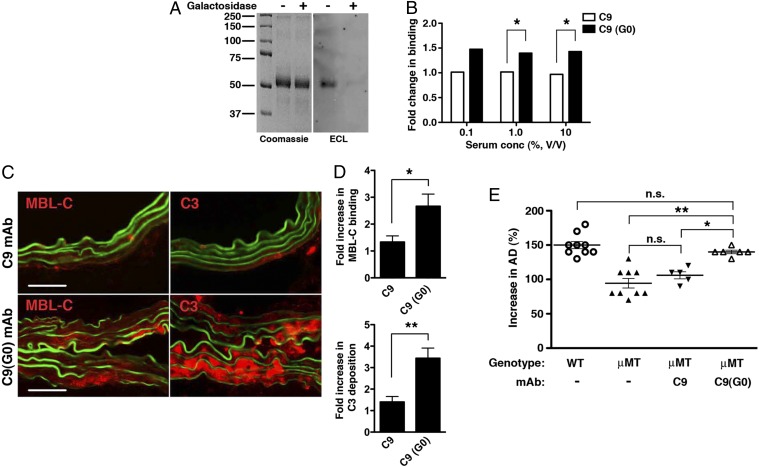
Fig. 5.
Effect of galactose removal on C9 mAb activity. (A) Untreated and β-galactosidase–treated C9 mAbs were blotted with Erythrina cristagalli lectin (ECL) to confirm depletion of terminal galactose residues in C9(G0). Coomassie blue-stained gel is shown for loading control. (B) Binding of MBL-C to untreated C9 and galactose-depleted C9(G0) mAb. Data shown represent mean ± SEM of triplicate values derived from two independent experiments. *P < 0.01. (C) μMT mice were perfused with elastase and administered 250 μg of C9 or C9(G0) mAb i.v. At 30 min, aortas were harvested and examined for MBL-C (red) or C3 (red) deposition as evidence of complement activation. Representative photomicrographs are shown. (Scale bar, 50 μm.) (D) Quantitative analysis of MBL-C and C3 deposition in abdominal aortas 30 min after elastase perfusion and mAb transfer. n = 3 aortas per treatment, six to nine sections per aorta. Values represent fold increase in integrated OD (IntDen) ± SEM compared with isotype control (set at 1). *P = 0.0089, **P = 0.0008. Pathogenicity of C9(G0) mAb in μMT mice. (E) Day 14 AD, expressed as percentage, in WT, untreated or μMT mice treated i.v. with 250 μg of the indicated mAb. Values represent mean ± SEM; each symbol represents an individual mouse. *P < 0.01, **P < 0.001, NS, not significant.