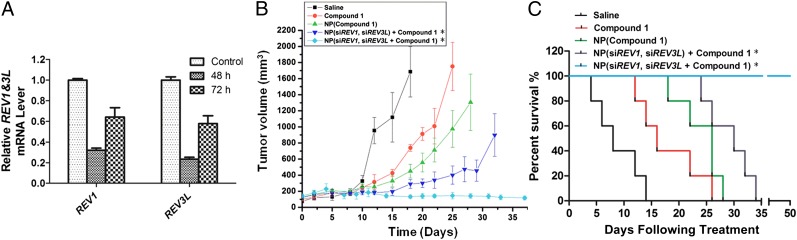
Fig. 5.
Enhanced in vivo therapeutic efficacy mediated by NP(siREV1, siREV3L, compound 1). (A) qRT-PCR confirmation of REV1 and REV3L gene suppression in LNCaP cells that were harvested from a xenograft tumor and isolated by GFP sorting 2 or 3 d after injection of NP(siREV1, siREV3L). (B) Inhibition of LNCaP xenograft tumor growth by formulation v [NP(siREV1, siREV3L, compound 1] in comparison to the following formulations: (i) saline, (ii) compound 1 in solution form, (iii) NP(compound 1), and (iv) NP(siREV1, siREV3L) with compound 1 in solution. The doses of compound 1 and siRNA per injection were 4 mg/kg and 0.4 mg/kg, respectively. (C) Survival curves of tumor-bearing mice treated with the aforementioned five formulations. Day 0 represents the first day of NP(siREV1, siREV3L) administration [n = 5 for groups i–iv, n = 8 for group v: P < 0.0136 for compound 1 vs. NP(siREV1, siREV3L) + compound 1; P < 0.008 NP(compound 1) vs. NP(siREV1, siREV3L, compound 1)]. P values for all survival studies were determined using log-rank curve comparison tests. *Before treatment with formulations iv and v, the tumor-bearing mice were injected on day 0 and day 2 with NP(siREV1, siREV3L). Starting from the fourth day, the mice received intratumoral injections of the aforementioned five formulations twice weekly. Day 0 represents the first day of NP(siREV1, siREV3L) administration.