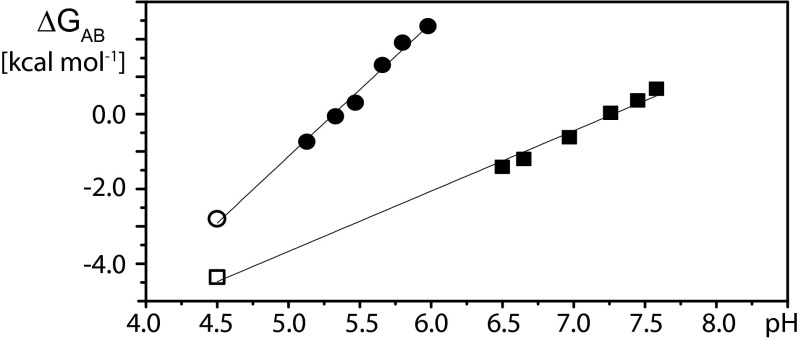
Fig. 4.
Dependence on pH of the Gibbs free energy difference between BmorPBPA and BmorPBPB, ΔGAB. Data derived from experimental measurements with delipidated aqueous solutions of BmorPBP measured at 20 °C are represented by squares for BmorPBP in the absence of bombykol and by circles for BmorPBP in the presence of equimolar bombykol. The values indicated by filled symbols were calculated using equilibrium constants determined with Eq. 3 from the pH titration data in Fig. 2. The values represented by open symbols were obtained with Eq. 2 from the amide proton exchange data in Fig. 3.