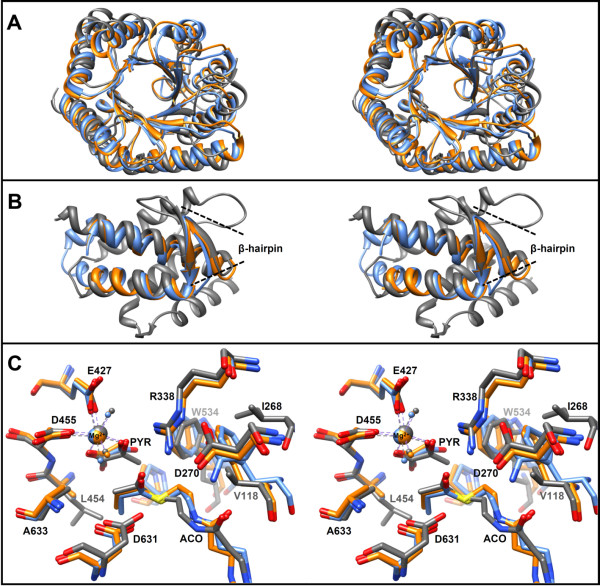
Figure 8.
Overlays of MCLC, MCLR, and MSGE. A) Stereo view of a superposition of only the central TIM-barrel secondary structure elements of MCLC (PDB 4L80), MCLR (PDB 4L9Y), and malate synthase G of the E. coli (PDB 1P7T). MCLC (blue), MCLR (orange), MSGE (gray). The overall rmsd between 212 Cα pairs is 2.0 Å. B) Stereo view of a superposition of the C-terminal lid domains. The rmsd between 42 Cα pairs is 1.4 Å. Only two of the α-helices as well as their connecting β-hairpins are structurally conserved between the MCLs and malate synthases. C) Stereo view overlay of active site residues and bound ligands (rmsd is the same as in A). Propionyl-CoA and oxalate are bound in MCLC, propionyl-CoA and glyoxylate are bound in MCLR, whereas acetyl-CoA (ACO) and pyruvate (PYR) are bound in MSGE. The numbering of residues corresponds to MSGE. A positionally conserved alanine residue in all malyl-CoA lyases is substituted by Leu454 in MSGE; it may sterically hinder propionyl-CoA and β-methylmalyl-CoA binding.