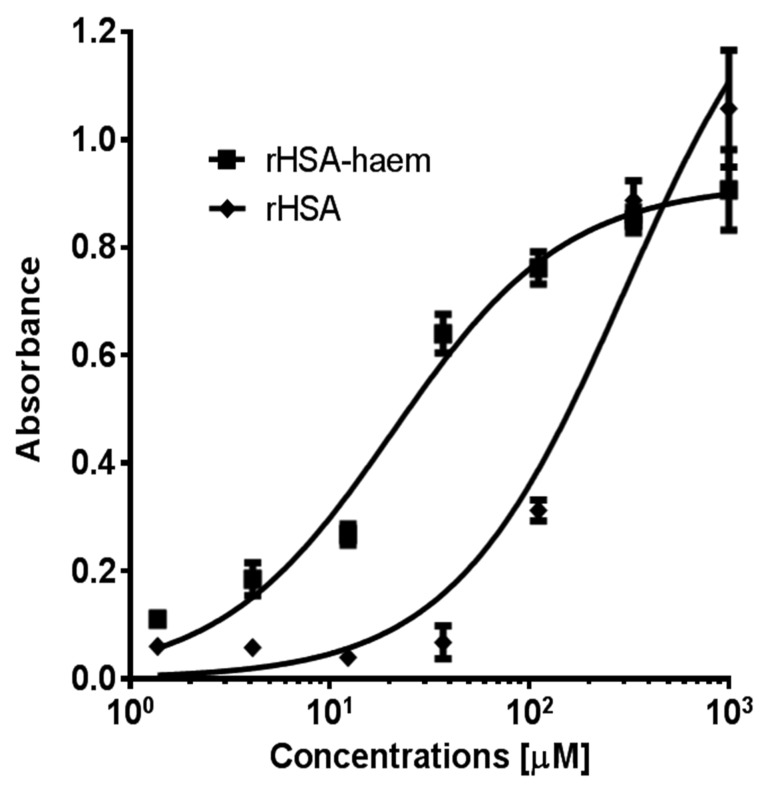
Fig. 4.
Interaction of rHSA and rHSA-haem with K1 polypeptide. 96-well ELISA plates were coated with K1 protein (0.4 μg/well in PBS) and incubated overnight at 4 °C. The wells were blocked with 1% skim milk in PBS for 1 h. Purified rHSA or rHSA-haem was added to the plate in various concentrations. Thereafter, anti-HSA mAb (15C7, AbCam) was added, followed by alkaline phosphatase-conjugated rabbit anti-mouse IgG. Colour development was detected with phosphatase substrate. Data were fitted by non-linear regression using GraphPad Prism 4.0 software (GraphPad, La Jolla, CA, USA). Apparent Kd value determined for binding of K1 to rHSA was 300 μM and for rHSA-haem, 20 μM. The fitted curves for rHSA and rHSA-haem with R2 values of 0.97 and 0.98, respectively