Abstract
Developmental methylmercury (MeHg) exposure produces response perseveration on discrimination reversal procedures, disrupts sensitivity to reinforcement, and enhances sensitivity to dopamine agonists – a profile suggesting a deficit in behavioral inhibition. To examine inhibition, we examined MeHg’s effects on the acquisition and persistence of low-rate lever-pressing following a history of high-rate responding. Additionally, we examined whether chronic exposure to selenium protects against MeHg’s developmental neurotoxicity. Female rats were exposed in utero via maternal exposure to drinking water containing 0 ppm, 0.5 ppm or 5 ppm of Hg as MeHg, producing approximately 0 μg/kg/day, 40 μg/kg/day, or 400 μg/kg/day of Hg. The mothers (during gestation) and the offspring (throughout life) consumed a purified diet containing 0.06 ppm or 0.6 ppm of Se (as sodium selenite), forming a 2 (lifespan diet) × 3 (developmental MeHg) factorial design. Adult offspring lever-pressed under two schedules of reinforcement. A differential reinforcement of high-rate (DRH) schedule imposed rigid response requirements that remained constant through the study. A high-rate percentile schedule (PCNT-H) incorporated a flexible criterion that reinforced short interresponse times using an adjusting criterion that was sensitive to recent performance. After high-rate responding stabilized, the PCNT-H schedule was abruptly inverted by reinforcing long interresponse times. Acquisition of low-rate responding was impaired in the MeHg-exposed rats because of intrusions of high-rate response bursts. DRH response rates did not change. Dietary selenium did not influence MeHg’s effects. High-rate operant behavior perseverated, suggesting that gestational MeHg exposure impairs response inhibition – an effect that extends results previously reported using choice procedures or spatial and visual discrimination reversals.
Keywords: Response inhibition, Reversal learning, Percentile Schedule, Methylmercury, Selenium, Developmental neurotoxicology
1. Introduction
The early development of the nervous system is vulnerable to adverse influences such as exposure to drugs or contaminants during early development. Such exposures can have long-term and irreversible consequences that last into adulthood or, in some cases, may be revealed only at an advanced age [1]. Methylmercury (MeHg), a potent developmental neurotoxicant, produces mental retardation, cerebral palsy, speech disorders, delayed walking, and motor dysfunction at high exposure levels [2–4]. The consequences of low-level exposure are more subtle and appear without such overt signs. In rodent models, maternal exposures that result in low micromolar brain concentrations in the developing offspring brain produce deranged and reduced outgrowth of neurites in the cerebral cortex of the offspring [5], neuroanatomical changes that suggest the possibility of behavioral consequences related to cortical damage.
Low-level MeHg exposure during gestation has significant and long-lasting behavioral consequences that include sensory-motor [6,7] and cognitive [8,9] domains. Young monkeys exposed to MeHg during gestation showed impaired choice between sources of rich or lean reinforcement rates. These changes were seen both during the transitional period that occurs after a change in the relative reinforcer rates as well as in steady state [10]. The effect was attributed to a disruption in the impact of reinforcing events. A similar outcome was observed in aged rats exposed during gestation to MeHg [11]. In other studies, adult rats exposed during gestation display perseveration and retarded transitions on a spatial [12,13] and visual [12] discrimination reversal – both tests of intradimensional shift. In order to accomplish a discrimination reversal, the previously reinforced response must cease, or be inhibited, and then responding must begin on a novel lever. Evidence that the inhibition of previously reinforced responding is impaired by gestational MeHg exposure is seen in prolonged extinction [14] and enhanced acquisition of fixed-ratio or progressive ratio responding [15,16]. Thus, behavioral effects of low-level MeHg appear consistently in procedures that tap resistance to change, reinforcement function, and behavioral flexibility, whereas memory effects are not consistently reported [6,14,15].
Perseveration along a motoric dimension can be studied by reinforcing a pattern of low-rate responding after first establishing responding at a high rate. High-rate responding is characterized by response bursts of relatively short interresponse times (IRTs) with intermittent pausing [16]. These bursts, or bouts, of short IRTs are resistant to change [16,17], which suggests that establishing high-rate behavior establishes a prepotent response pattern. Motoric perseveration would appear as the persistence of short IRTs under conditions that reinforce low-rate responding, providing evidence of behavioral rigidity in a dimension other than those tapped by spatial or visual discrimination reversals. The successful acquisition of low-rate responding would then require inhibiting intrusive, prepotent response patterns characteristic of high-rate behavior. Response inhibition, one of the executive functions, is important because it prevents previously reinforced behavior from interfering with the acquisition of new behavior. There is neurochemical evidence that inhibitory processes are disrupted by gestational MeHg exposure. Such exposure diminishes activity of the inhibitory neurotransmitter GABA [18,19] and in behaving animals decreases sensitivity to pentobarbital, which activates the inhibitory GABA-Chloride ionophore, [20].
High response rates can be generated by selectively reinforcing interresponse times (IRTs) using either a differential reinforcement of high-rate (DRH) or a high-rate percentile (PCNT-H) schedule of reinforcement [21–23]. For example, in a DRH 8:4 schedule, an 8-response burst of lever-presses must begin and end within 4 s to be eligible for reinforcement [24]. The DRH schedule imposes a direct relationship between motor ability (the ability to produce criterion 8-response bursts) and reinforcement rate. In contrast to the DRH, the PCNT-H schedule uses a titrating criterion IRT. For example, under a PCNT-H 20:0.75 schedule, each IRT is compared with the previous 20 IRTs and is eligible for reinforcement (and produces a conditioned reinforcer) if it is shorter than 75% of them. Thus, high response rates are maintained but the IRT criterion continuously adjusts according to recent performance. To stabilize overall reinforcement rate, criterion IRTs can be reinforced with sucrose randomly at an average rate of 2 reinforcers per minute (Random Interval 30″ schedule). Holding reinforcement rate constant across a large range of response rates ensures that reinforcement rate remains steady for exposed animals until impairment becomes quite severe. By weakening the link between response and reinforcement rate, such a second-order percentile schedule avoids confounding the effects of reinforce loss with motor deficits [25,26].
To produce low-rate responding it is necessary only to invert the percentile contingency so that IRTs have to be longer than 75% of the previous 20 IRTs. This can be designated a low-rate percentile schedule (PCNT-L). Because the PCNT-L has no natural minimum interresponse time, animals can acquire low-rate responding at their own pace and without loss of reinforcement. Therefore, it is possible to examine both the rate of change from a high-rate to a low-rate contingency and the level of asymptotic performance with percentile schedules.
The element selenium is protective against neurotoxicity caused by adult-onset MeHg exposure [27–34] as well as its reproductive toxicity [30,35]. This protection could be due to a tight bond formed between mercury and selenium that reduces the bioavailability of both elements. Reducing MeHg’s bioavailability would also reduce its neurotoxicity, but reducing selenium’s availability could adversely impair normal selenoenzyme activities or reduce its antioxidant effects [30,35]. It has been hypothesized that the mercury–selenium bond should mitigate MeHg’s developmental neurotoxicity, too [36,37] but this hypothesis has not been supported by in vivo studies (reviewed in Section 4). The present study was designed to examine the acquisition of low-rate responding in rats exposed gestationally to MeHg and whether a lifelong diet that is rich in selenium can protect against deleterious effects of exposure.
2. Methods
2.1. Subjects
The subjects were 33 adult female Long-Evans rats (4–7 per experimental group) bred in the laboratory. They were siblings of rats reported by Newland, Reed, and colleagues [13,39,41,38]. Each was randomly selected from a separate litter, so the litter served as the statistical unit. The breeders were purchased from Harlan (Indianapolis, IN). The subjects were weaned at postnatal day 21 and at this time were injected subcutaneously with an electronic identification chip (Biomedical Data Systems, Seaford, DE) to enable individual identification throughout life. They had ad libitum access to food until they reached a body mass of 240 g, at which point their food was rationed to approximately 10 g/day so as to maintain their body mass at 240–260 g. Rats had ad libitum access to tap water in their home cages and were housed two per cage in standard rat cages divided in half along the diagonal with a clear plastic divider. The colony was housed in an AAALAC-accredited facility in a temperature- and humidity-controlled room on a 12-h light–dark cycle (lights on at 7:00 a.m.). Animal health was monitored daily by research staff, with weekly inspections by veterinary staff. All procedures were approved by the Auburn University Institutional Animal Care and Use Committee.
2.2. Selenium and MeHg exposure
The subjects were exposed in utero to 0 ppm, 0.5 ppm, or 5 ppm Hg as methylmercuric chloride via maternal drinking water and to 0.06 ppm or 0.6 ppm Se via maternal diet according to a 2 × 3 factorial design. The diet was based on the AIN-93 purified formulation (Research Diets Inc., New Brunswick, NJ). Breeders began dietary exposure at 18 weeks of age, 5 weeks before breeding began. Maternal MeHg exposure continued to post-natal day 16 but since there is little MeHg exposure via milk we conclude that offspring exposure ended at birth [39]. The offspring ceased MeHg exposure at birth but continued their Se diet throughout life. The low-selenium diet contained Se from casein at a nominal concentration of 0.06 ppm and measured concentrations ranged from 0.05 ppm to 0.07 ppm. The high-selenium diet was supplemented with sodium selenite resulting in a nominal Se concentration of 0.60 ppm and measured concentrations were 0.6–0.9 ppm. Although the diets are labeled as “low” and “high” selenium, both were within the healthy range of selenium content so neither selenium deficiency nor toxicity would occur. Between mating and lactation, the base diet for the breeders was an AIN-93 growth diet containing 7% fat from soybean oil. A maintenance diet of an AIN-93 diet with 4% fat was used at all other times. Dietary mercury was below the detectable level of 50 ppb. Male breeders were maintained on the chow diet, except when briefly exposed to the female’s diet during breeding. All offspring received the same diet as their maternal dams throughout life.
At 21 weeks of age, after three weeks on the custom selenium diet, each selenium group of breeders was subdivided into three MeHg exposure groups to produce six experimental groups. MeHg was added to the drinking water at concentrations of 0 ppm, 0.5 ppm, or 5.0 ppm of mercury as methylmercuric chloride (Alfa Aesar, Ward Hill, MA). These concentrations produce approximately 0 μg/kg/day, 40 μg/kg/day, and 400 μg/kg/day of mercury consumption, respectively, based on average daily consumption, but with elevation of exposure late in gestation due to increased fluid consumption [40]. Maternal exposure to the MeHg-containing water terminated on post-natal day 16 when the offspring could reach the drinking water spout. From this point on, the offspring drank tap water with no MeHg added.
Brain mercury concentration at birth for siblings of the animals used in the present study are reported elsewhere [41]. For the 0 μg/kg/day, 40 μg/kg/day, and 400 μg/kg/day (control, 0.5 ppm and 5 ppm) groups they were nondetectable, 0.2 ppm or 5 ppm (nondetectable, 1 μM or 20 μM) in the brain. The limit of detection was 50 ppb. Brain selenium ranged from 0.08 ppm to 0.11 ppm (1–1.4 μM) depending upon dietary selenium and MeHg exposure.
2.3. Breeding
Breeding commenced at approximately 23.5 weeks of age, after 2.5 weeks of maternal MeHg exposure. Each male was mated with two females and mating continued until body-weight increased, suggesting gravidity. Births before 5:00 p.m. were assigned to PN 0 for that day. All births after 5:00 p.m. were assigned to PN 0 for the next day. Large litters were culled and small litters were combined to produce eight pups per litter. Only one female per litter was included in the present study.
2.4. Behavioral methods
Experimental sessions were conducted in commercial rodent operant chambers (Med-Associates, Model ENV-008) configured with two sonalert tones (nominally 2900 Hz and 4500 Hz, calibrated to 70 dB), a house lamp (28 V 100 mA), one (unused) lever on the rear wall and two retractable levers on the front wall. All levers were calibrated to 0.20 N to operate. A pellet dispenser stocked with 45 mg precision sucrose pellets (Research Diets Inc., New Brunswick, NJ) was situated between the two front levers; and an LED was above each lever. Each chamber was situated inside a sound-attenuating cabinet, with a built-in exhaust fan that also served to mask extraneous noises. Session events were recorded with decisecond resolution via MED-PC IV software (Med-Associates, St. Albans, VT) running on a PC with the Windows NT operating system. Programs that controlled experimental sessions and data collection were written in Medstate Notation. Sessions were conducted 5 days/week and 45 mg sucrose pellets were used as reinforcers. The reinforcement cycle included a tone (Sonalert, nominally 4.4 kHz) that followed criterion responses, then a delivery of a sucrose reinforcer.
2.4.1. PCNT-H 20:0.75: high-rate schedule
Lever-pressing was autoshaped in overnight sessions and then placed under the PCNT-H schedule of reinforcement. Immediately after autoshaping the rats responded under a PCNT-H 10:0.5 schedule, in which an IRT met criterion for reinforcement if it was shorter than 50% of the previous 10 IRTs and criterion IRTs were reinforced under an RI 30″ schedule. After about 50 sessions this became a more demanding, PCNT-H 20:0.75 schedule, in which an IRT was eligible for reinforcement if it was shorter than 75% of the previous 20 IRTs. Every criterion IRT was followed by the same 0.2″ tone (Sonalert©), nominally 4.4 kHz) that accompanied sucrose pellet delivery.
Under the PCNT-H 20:0.75 schedule, each lever-press was recorded and the interresponse time between lever-presses was stored in a 20-element array. When a lever-press terminated an IRT, that IRT was compared with the previous 20. If the IRT was shorter than 75% (15) of the previous 20 then it was flagged as eligible for reinforcement. Regardless of whether that IRT met criterion, it was then stored in the array and the most distal IRT pushed out, thus sliding the “look-back” window forward. Subsequent IRTs were treated similarly. The RI 30″ schedule was arranged by querying a probability gate every second and, if true, then the next criterion response resulted in sucrose delivery. Thus, criterion IRTs were reinforced at unpredictable times but at an average rate of 2 reinforcers per minute.
2.4.2. PCNT-L 20:0.75: low-rate schedule
The low-rate schedule worked similarly to the high-rate schedule except that to be eligible for reinforcement an IRT had to be longer than 75% of the previous 20 IRTs.
2.4.3. Differential reinforcement of high-rate (DRH 8:4) schedule
For final performance, a criterion response burst consisted of 8 responses within 4 s. As with the percentile schedule, responding was monitored continuously (i.e., this was not a trials procedure). Thus, when a lever-press was recorded the total time, T, elapsed between that press and the 7th prior lever-press had to be less than 4 s to meet criterion. Following each criterion response burst, the 0.2″ tone that accompanied reinforcer delivery was delivered and every fifth criterion burst resulted in delivery of primary reinforcement (a sucrose pellet). Technically, this can be called a second order FR 5 (DRH 8:4) schedule of reinforcement. The DRH schedule was unchanged through the course of the experiment.
2.4.4. Training, baseline, and alternation of percentile and DRH schedules
To establish DRH performance, lever-pressing was initially placed under a DRH 2:1 (two responses within 1 s) immediately after autoshaping. This was gradually increased to a DRH 8:4 via a series of intermediate steps (DRH 4:2, DRH 6:3, and finally DRH 8:4 schedule) over the course of 32 sessions.
The percentile schedule is essentially a shaping schedule as it adjusts its criterion according to an animal’s responding, so there was little need to introduce it gradually. As noted in Section 2.4.1, training began with a PCNT 10:0.5 schedule and this was changed to a Percentile 20:0.75 schedule to generate higher response rates. The final schedule, Multiple FR 5(DRH 8:4) RI 30″ (PCNT 20:0.75) schedule was in effect for 50 sessions before the low-rate percentile was introduced. The low-rate percentile remained in-place for 29 sessions and the DRH schedule was unchanged during this low-rate challenge. Then the high-rate percentile was reinstated and six sessions were used to examine the return the high-rate schedule.
Throughout all phases of the experiment, the percentile and DRH schedules were presented in strict alternation (with the percentile always occurring first within a session) as a multiple schedule with 2-min components that were separated by a 20″ blackout throughout the course of a 28′ session, of which 24′ was non-blackout time available for responding. An overhead, 28-V houselight was turned on during the PCNT component and off during the DRH component. Presenting these as a multiple schedule permitted the direct, within-subject comparison of response pattern during a single session, and permitted an assessment of discriminative control over response patterns by the two schedules in effect.
2.5. Data analysis
2.5.1. Statistical analysis of transitions
The primary behavioral measure was the median IRT under the DRH and the PCNT-H or PCNT-L components for a single rat during a session. For one subject in the 5.0 ppm MeHg exposure group, two sessions’ data during the low-rate challenge were excluded from analyses of PCNT-L responding. During those sessions, the subject’s median IRTs were 3.9 and 3.6 standard deviations above the group mean, and were 10.2 and 9.6 times higher than that subject’s own median IRT calculated from a span of 7 consecutive sessions that included both aberrant sessions.
The change in median IRT that occurred after the inversion of the PCNT-H schedule to a PCNT-L schedule was modeled with a four parameter logistic function:
| (1) |
where YBL is the left asymptote (baseline), YSS is the right asymptote. Xhalf is the session number at which ½ of the transition had occurred (hereafter the “half-max”). The half-max locates the S-shaped function along the horizontal axis and designates the inflection point, i.e., the point at which the rate of change begins to slow. The parameter, a, specifies the number of sessions required to complete 1/e–2/e (approximately 1/2.7–2/2.7) of the transition once it begins and captures the maximum rate of change. Fits were performed for each subject using nonlinear least squares in R [42]. The resulting parameter estimates were aggregated within exposure groups and analyzed as a factorial ANOVA with diet and MeHg exposure treated as between-group factors.
2.5.2. Interresponse time (IRT) distributions
IRT histograms from individual rats and group averages of cumulative distributions of IRTs were generated from three sessions in order to characterize the effect of the low-rate challenge on response pattern in the three exposure groups. These two displays are included to emphasize different features of the distributions of IRTs during the low-rate challenge. Cumulative distributions are shown as group averages for the last session (N-1) preceding the low-rate challenge, the 18th session (when acquisition was about ½ complete) and the last session. All IRTs from the percentile and, separately, the DRH components were accumulated, sorted, and the resulting distributions were divided into 20 quantiles. The average value and standard error of the mean of each of the 5th, 10th, … 95th quantiles were calculated for all rats in the same exposure group and then expressed as cumulative distributions. Uncumulated distributions from individual rats are shown for the last session of the high-rate percentile schedule, and session and the last session of the low-rate percentile schedule.
2.5.3. Bout analysis
We conducted a log-survivor analysis to quantify the bout structure of responding and its changes during the low-rate challenge. This analysis partitions IRTs into two distributions: a within-bout distribution made up of short IRTs from high-rate response bursts, and a between-bout distribution made up of long IRTs associated with pausing between bouts [16]. The model’s parameters are differentially sensitive to drug effects and motor and motivational influences [16,25]. Consistent with the goal of quantifying response perseveration, the present analysis focuses on changes in the length of response bouts.
This approach models the IRT distributions as a biexponential mixture:
| (2) |
where yt is the proportion of IRTs greater than or equal to t (s). The mixing parameter, p, is the proportion of IRTs in the within-bout distribution, b is the rate at which bouts are initiated and w is the rate of responding within bouts (both b and w are in responses/s). The average number of lever-presses in a bout is 1 + 1/(1 − p). Adding 1 is necessary to convert IRTs (which comprise two responses) to lever-presses. For details see [16,43,25].
The bout analysis was conducted on PCNT-H and PCNT-L responding for all sessions from baseline through reacquisition of high-rate responding. Because there were few IRTs and few response bouts for most subjects during the final sessions of the PCNT-L condition, the last 5 sessions were excluded from analysis. We used the same 4-parameter logistic model described in Section 2.5 to model the changes in p over time.
3. Results
3.1. Percentile component
A 2 × 3 factorial ANOVA conducted on the logistic parameters revealed no significant selenium by mercury interactions (YBL: F(2, 27) = 0.81, p = 0.45; YSS: F(2, 27) = 2.41, p = 0.11; Xhalf: F(2, 27) = 0.16, p = 0.85; a: F(2, 27) = 0.63, p = 0.54), and no significant main effects of selenium (YBL: F(1, 27) = 1.57, p = 0.22; YSS: F(1, 27) = 1.06, p = 0.31; Xhalf: F(1, 27) = 0.01, p = 0.93; a: F(1, 27) = 2.48, p = 0.13) on any of the logistic parameters. Therefore, data were collapsed across diet groups and only MeHg-related effects are described below.
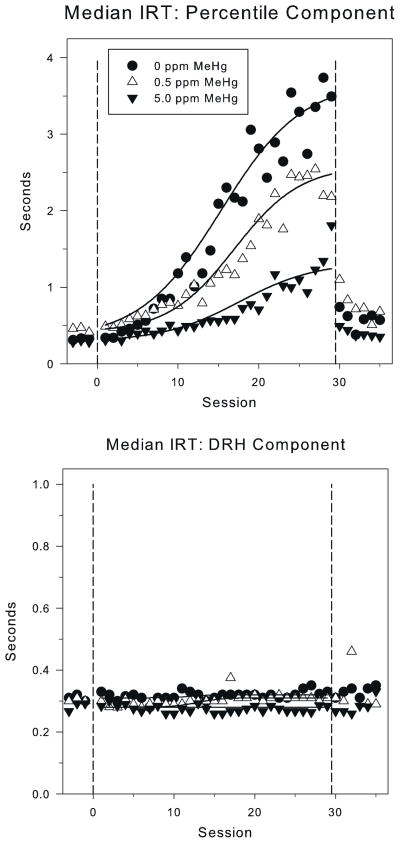
Fig. 1 (top panel) shows the median IRT with best-fit logistic function during the PCNT-L component for the three MeHg exposure groups. During the baseline sessions prior to the low-rate challenge, the median IRT stabilized at 0.35″–0.4″, corresponding to response rates of 2.5–3.3 responses per second for all exposure groups. There were no significant MeHg group differences in the baseline asymptote, YBL (0.33, 0.39, and 0.30 for the control, 0.5 ppm, and 5.0 ppm exposure groups, respectively): F(2, 27) = 2.02, p = 0.15.
Fig. 1.
The mean of the median interresponse times (IRT) under the high- and low-rate percentile (PCNT-H, sessions less than 0 and greater than 30, and PCNT-L, sessions 1–29) (top panel) and the DRH schedules (bottom panel) for each of the MeHg exposure groups. The solid lines show best-fit lines from the 4-parameter logistic model (Eq. (1)). The dashed vertical lines mark the phase changes at x = 0 (from PCNT-H to PCNT-L) and x = 30 (from PCNT-L to PCNT-H). Note the scale difference for the y-axis between the top and bottom panels.
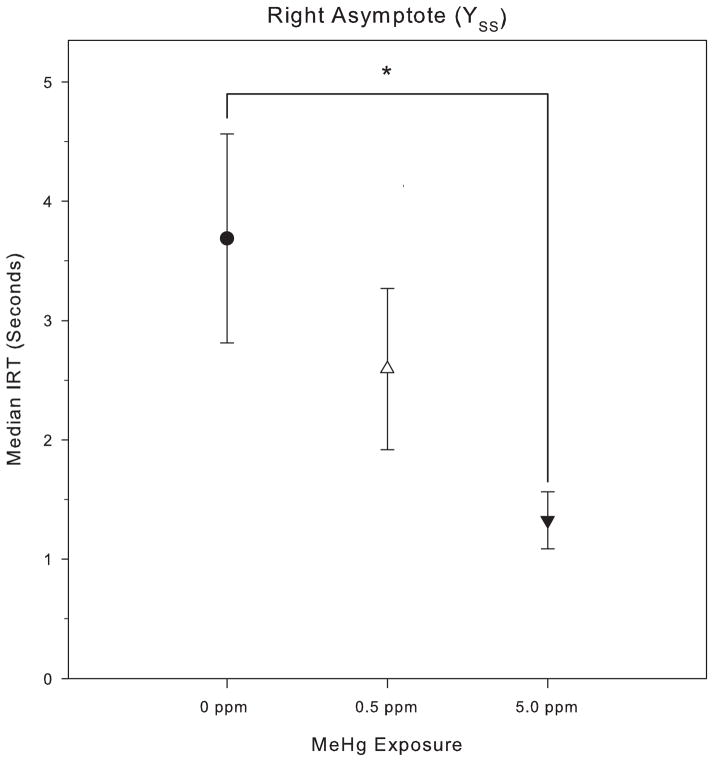
For session 1 through 29 of the PCNT-L 20: 0.75 schedule, the median IRT increased in all groups. The asymptotic median IRTs (YSS) for the control group, 0.5 ppm and 5.0 ppm MeHg groups were 3.8 s, 2.8 s, and 1.3 s, respectively (Fig. 2); these IRTs correspond to response rates of 0.26 responses/s, 0.36 responses/s, and 0.77 responses/s. An ANOVA revealed a significant effect of MeHg exposure on the right asymptote, YSS, F(2, 27) = 4.25, p = 0.02). Post hoc testing via Tukey’s HSD showed a significant difference in the right asymptotic median (Fig. 2) between the 5.0 ppm MeHg exposure group and the 0 ppm control group, but the 0.5 ppm group was not statistically distinct from either group. In relative terms, the control group showed a roughly 12-fold increase in median IRT, the 0.5 ppm MeHg group showed a roughly 9-fold increase, and the 5.0 ppm showed a roughly 4-fold increase. There were no significant MeHg group differences on half-max (control: 17.0 ppm, 0.5 ppm: 17.6 ppm, 5.0 ppm: 20.4), Xhalf: F(2, 27) = 0.64, p = 0.53, nor rate (control: 5.9 ppm, 0.5 ppm: 6.4 ppm, 5.0 ppm: 6.0), a: F(2, 27) = 0.42, p = 0.67). Thus, gestational MeHg exposure muted subjects’ maximal reaction to the low-rate challenge without affecting either the latency to transition, or the rate of change.
Fig. 2.
Mean (with SEM) of the right asymptotic median, YSS, for responding on the PCNT schedule during the low-rate challenge phase.
After the PCNT-H schedule was re-imposed, high-rate responding recovered quickly for control and exposed animals, with no significant main effects of MeHg (YBL: F(2, 26) = 0.78, p = 0.47; YSS: F(2, 26) = 0.91, p = 0.42; Xhalf: F(2, 26) = 0.11, p = 0.89; a: F(2, 26) = 0.01, p = 0.99), or Se (YBL: F(1, 26) = 1.14, p = 0.30; YSS: F(1, 26) = 0.24, p = 0.63; Xhalf: F(1, 26) = 0.25, p = 0.62; a: F(1, 26) = 0.33, p = 0.57), and no MeHg by Se interactions (YBL: F(2, 26) = 0.35, p = 0.71; YSS: F(2, 26) = 0.46, p = 0.63; Xhalf: F(2, 26) = 1.90, p = 0.17; a: F(2, 26) = 1.40, p = 0.26).
Throughout the PCNT-L challenge and the reacquisition of the PCNT-H, the overall reinforcement rate remained relatively stable at 1.20–1.60 reinforcers/min for all groups. There was no significant effect of MeHg or Se on reinforcement rate, indicating that the exposure-dependent differences in the median IRT were unrelated to reinforcement rate.
3.2. DRH component
The bottom panel of Fig. 1 shows the median IRT during the DRH 8:4 schedule for the same time window as the top panel of Fig. 1. The median IRT was stable for all groups with a mean of approximately 0.3 s, corresponding to a response rate of 3.33 responses per second, throughout the low-rate (PCNT-L) challenge. There were no significant main effects or interactions on any of the logistic parameters for DRH responding during the low-rate challenge or the reacquisition of high-rate (PCNT-H) responding (all F’s < 1.0). At no time were there significant effects of exposure or session on the median IRT under the DRH schedule. However, there was a significant MeHg effect (F(2, 390) = 4.46, p = 0.01) on DRH reinforcement rate, but no session by MeHg interaction (F(2, 390) = 1.06, p = 0.35). The DRH reinforcement rate for each group was stable throughout the low-rate challenge, with the 5.0 ppm MeHg group having slightly higher (4.50–4.75 reinforcers/min) reinforcement rate than the control and 0.5 ppm MeHg groups (3.75–4.25 reinforcers/min).
3.3. IRT analysis
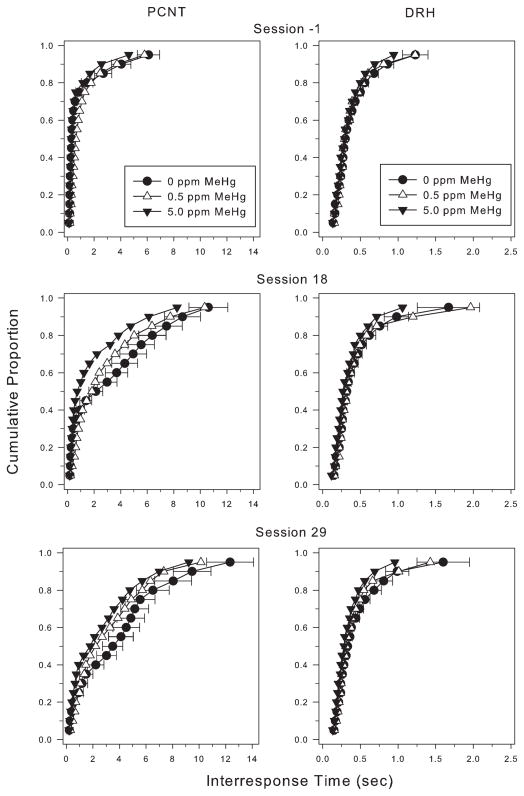
Cumulative IRT distributions from sessions -1 (last day of baseline), 18, and 29 for the PCNT-L and DRH components are shown in Fig. 3 (left and right panel, respectively) as group averages. At session -1, the IRT distributions from all three exposure groups (Fig. 3, top left panel) were similar to one another. Responding was characterized by high-rate response bouts; more than 75% of the IRTs were shorter than 1 s and relatively few represented pauses in responding. For controls, the PCNT-L schedule substantially changed the shape of the IRT distribution by increasing the duration of IRTs in the upper half to 2/3 of the IRT distribution by session 18 and increasing the duration of the longest 70% of the IRTs by session 29. The shape of the distribution from the 5 ppm group changed, too, but less so. As compared with controls and (for session 18) the 0.5 ppm group, there were fewer long IRTs and substantially more very short IRTs, representing high-rate bursts of responding with only infrequent or short pausing. Note that the IRT distributions from the DRH component (Fig. 3, right column) remained stable during this phase of the experiment.
Fig. 3.
Cumulative interresponse time (IRT) distributions from the PCNT-L (left column) and DRH (right column) components at selected sessions during the low-rate challenge. Note the scale differences on the x-axis for the left and right panels. Most IRTs were short at the beginning of this series of sessions and grew as long IRTs were reinforced. Exposed rats showed more short IRTs during this period than unexposed rats. In the right (DRH) column, IRT distributions for all three exposure groups remained relatively stable across the time period of the low-rate challenge. Note the differences in x-axis between left and right columns.
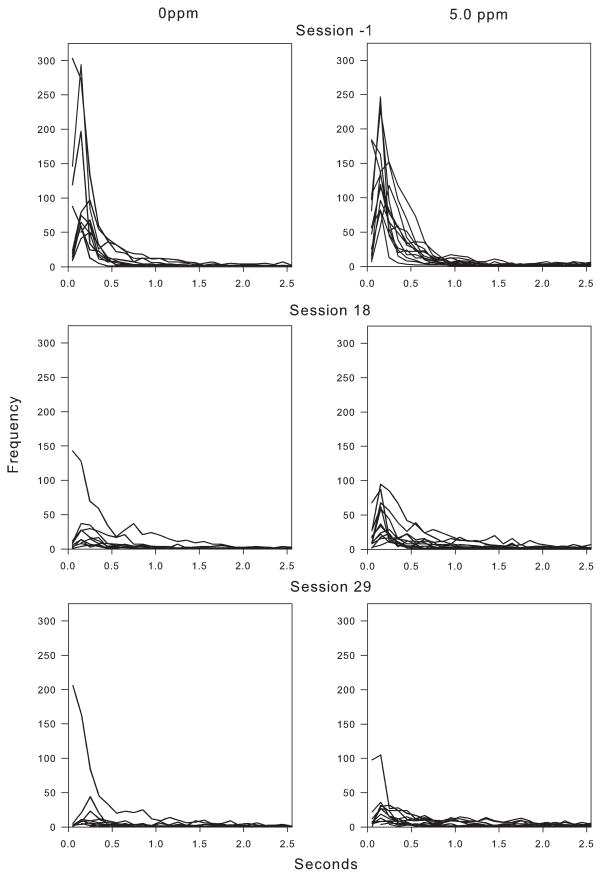
Uncumulated IRT distributions (Fig. 4) for the 0 ppm and 5 ppm exposure groups clearly show that the changes in responding over time were driven by a reduction in the frequency of short IRTs. For the last session of the high-rate schedule (top panels), there were many short IRTs and few long ones. For controls, the number of short IRTs plummeted by session 18 and continued to fall through session 29 (with a single exception). Exposed rats continued to show a relatively large number of short IRTs at session 18, although this continued to fall through session 29. Note the relative absence of high-rate response bursts over time on the PCNT-L schedule.
Fig. 4.
Uncumulated IRT histograms for individual rats from selected sessions of the PCNT-H and PCNT-L schedules for the 0 ppm and 5 ppm MeHg exposure groups. Session N-1, rather than session 1 of the PCNT-L, is shown because some behavioral adaptation occurred in the first session of the PCNT-L. For the 0 ppm exposure group, there is a marked, general decrease in responding (as would be expected with the decline in response rates) across sessions. The lost responses come primarily from the short IRT range. IRTs for the 5 ppm exposure group follow a similar pattern, but with a lesser reduction short IRTs.
3.4. Bout analysis
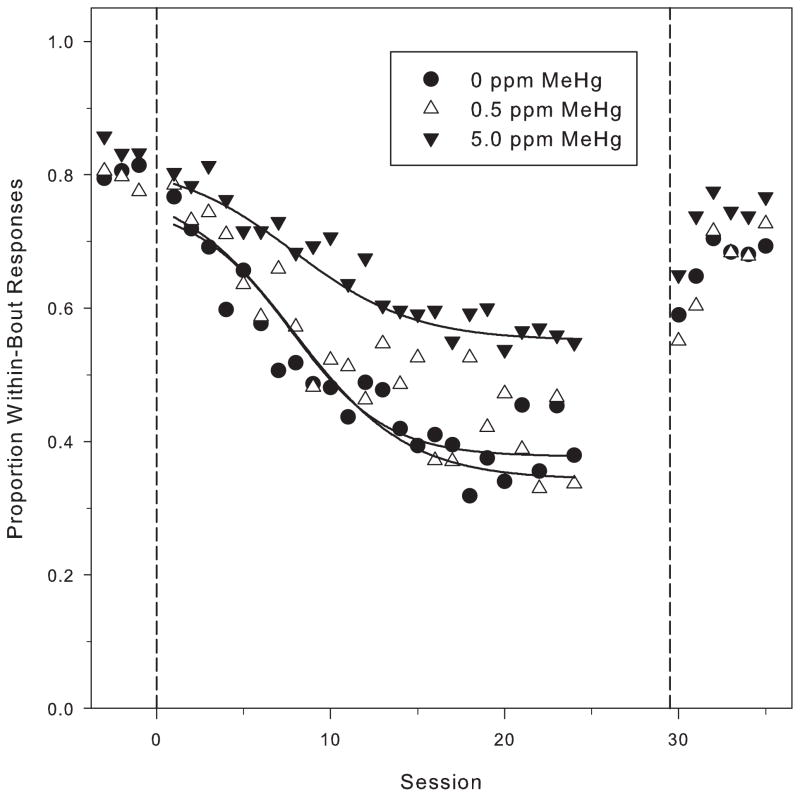
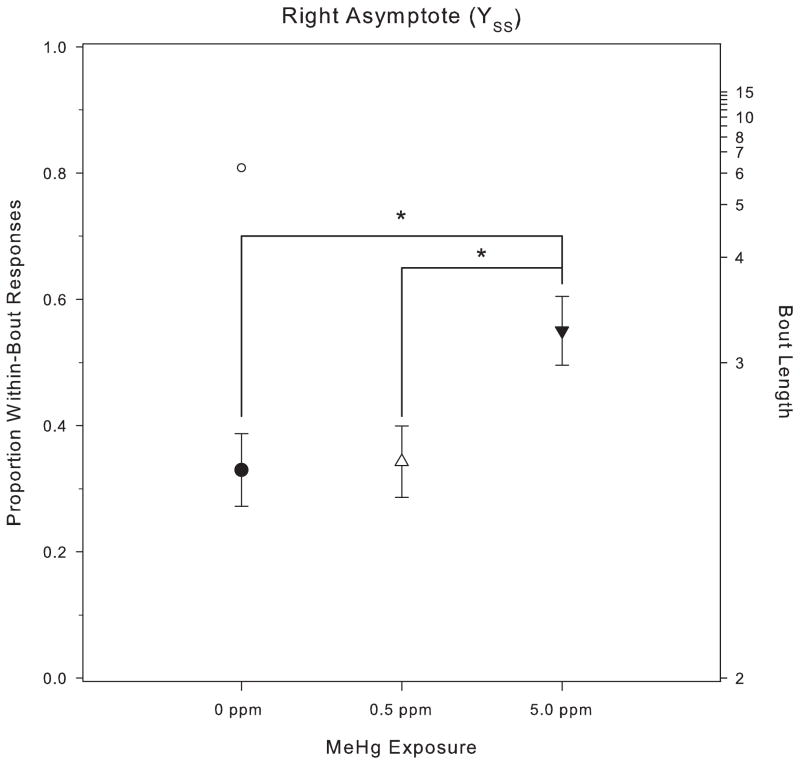
Fig. 5 shows p (the proportion of responses within a bout) plotted with the best-fit logistic function (Eq. (1)) for the three MeHg exposure groups on the PCNT schedule. During baseline, before the low-rate challenge was imposed, p, had stabilized at approximately 0.8 for all exposure groups. This corresponds to a bout length of about six responses. There were no significant differences in the baseline values of p among MeHg exposure groups (YBL: F(2, 29) = 1.50, p = 0.24). Across PCNT-L sessions, the mean of p declined for each of the three MeHg exposure groups, with the total change being greater for the 0 ppm and 0.5 ppm groups (approximately a 50% reduction from baseline) than for the 5.0 ppm group (approximately a 25% reduction from baseline). This difference across exposure groups in YSS, the asymptotic value of p (see Fig. 6), was significant (YSS: F(2, 29) = 3.63, p = 0.039). Post hoc testing via Tukey’s HSD revealed a significant difference between the 5 ppm and 0.5 ppm exposure groups. The value of YSS for one control subject (clearly visible in the bottom left panel of Fig. 4) was 2.8 standard deviation units above the group mean. When that subject was excluded from the analysis, Tukey’s HSD showed a significant difference between the 5 ppm and 0 ppm groups as well. Fig. 6 shows the right asymptote for the three groups, with the outlier shown separately.
Fig. 5.
The mean of the proportion of IRTs classified as within-bout IRTs (p) by log-survivor analysis during the PCNT-H and PCNT-L conditions. PCNT-L data from session 25 through session 29 are not plotted due to the difficulty achieving model fits with relatively few IRTs. The solid lines show best-fit lines from the 4-parameter logistic model (Eq. (1)). The dashed vertical lines mark the phase changes at x = 0 (from PCNT-H to PCNT-L) and x = 30 (from PCNT-L to PCNT-H) Note the scale difference for the y-axis between the top and bottom panels.
Fig. 6.
Mean (with SEM) of the right asymptote, YSS, for p on the PCNT schedule during the low-rate challenge phase. The single data point above the 0 ppm MeHg group represents the anomalous control subject seen in figure the bottom left panel of Fig. 4.
Neither the rate of change, a, nor the half-max value of the logistic differed significantly across groups (a: F(2, 29) = 0.027, p = 0.973; Xhalf: F(2, 29) = 2.04, p = 0.14). This decline in proportion of IRTs classified, as part of high-rate bursts is consistent with the decline in median IRT value described above. The differences in p at the end of the PCNT-L manipulation suggest that the high-rate response bouts were less sensitive to the manipulation of the temporal contingency for animals in the 5.0 ppm exposure group. Thus, to a greater extent than for the 0 ppm and 0.5 ppm groups, high-rate response bursts persisted for the 5.0 ppm group even after such responding became disadvantageous.
4. Discussion
Gestational MeHg exposure produces behavioral rigidity and altered sensitivity to reinforcing events as evidenced by retarded acquisition of choice [10,11], enhanced responding during extinction [44], perseverative responding and increased errors of commission in reversal learning procedures [12,13]. The results from the present study indicate that this resistance to change also appears in a very different behavioral dimension, the pace and structure of responding, but the processes tapped may be similar. The difficulties that the animals had in acquiring low-rate responding may have been due to an intrusion of prepotent responding, a component of diminished inhibitory control over behavior.
The present study can be compared to previous studies of spatial and visual discrimination reversals. In a spatial discrimination reversal, responses on one lever (left, say) are reinforced. When responding stabilizes, the source of reinforcement shifts to the other lever. Thus, two levers are spatially distinct and the spatial discrimination originally formed on the left is reversed. The first reversal is especially sensitive to interventions that inhibit the pre-frontal cortex [45,46]. In a visual discrimination reversal, responses on a lever located underneath a lit light are reinforced and, when behavior stabilizes, a reversal is imposed such that only responses under an unlit light are reinforced. Each type of reversal represents an intradimensional shift, because the relevant dimension (spatial location or illumination) is unchanged but which stimulus is associated with reinforcement changes [45,46]. Both spatial and visual discrimination reversals are sensitive to gestational MeHg exposure and the effect is most pronounced on the first reversal [12,13].
The present study extends the previous findings by demonstrating diminished sensitivity to a reversal of a motoric response requirement. Rats exposed to MeHg in utero were slower to adjust when the percentile requirement was changed from a high-rate to a low-rate (short IRT to long IRT) criterion. This transition can be construed as a reversal-learning procedure since initially short IRTs were reinforced and later long ones were reinforced. Here, the dimension is the time between responses rather than lever location or illumination. The return to a high-rate percentile schedule was not sensitive to gestational MeHg exposure. This might reflect the “first-reversal effect”, namely, that deficits in reversals are not seen, or are notably smaller, as the animal experiences more reversals, a sort of “learning to learn” phenomenon. A test of this hypothesis could be undertaken by training low-rate responding first and then introducing a high-rate response requirement.
MeHg’s effects represent an excessive impulsive-like intrusion of prepotent response bouts that were revealed in the transition from a high-rate to a low-rate schedule. Successful responding on such low-rate schedules requires that the animal press the lever, do something else for a period of time, and then press the lever again [47,48]. The history of high-rate responding in the high-rate percentile (here) and with a fixed-ratio schedule before introducing a DRL schedule (in [49]), may have established high-rate response bouts as a prepotent response that is inconsistent with low-rate responding. Thus, both the DRL schedule the low-rate percentile schedule tap at least two of the three processes that form the construct of response inhibition [50,51]: the protection of a delay period from competing events or responses that might occur and shorten that period of non-responding and the suppression of pre-potent responses.
It is well-established that high-rate, short-IRT, response bouts are characteristic of behavior under the DRL schedule [52–54] but they are apparently not characteristic of responding under the Percentile-L schedule in unexposed rats. The IRT histograms (Fig. 4) revealed that, for controls, few such bouts occurred with the low-rate percentile schedules at asymptote. Such bouts were more likely to occur in the exposed animals, as is evident by the steep rise in the cumulative IRT distribution (Fig. 3) and in the large number of short IRTs in the uncumulated histograms containing individual subjects (Fig. 4) at session 18 and, to a less extent, session 29. Thus, extinction of the high-rate response pattern was more prolonged, and these high-rate response bursts were more intrusive, for the exposed animals.
A history of explicitly reinforcing a pattern of high-rate response bouts can have long-lasting residual effects, resulting in higher response rates and shorter IRTs when responding is maintained by time-based schedules of reinforcement in laboratory animals [55–58] as well as in humans. [59,60]. In rats, a history of high-rate responding produces an elevation of bouts of high-rate responses under DRL schedules, suggestive of impulsive behavior [61] and can influence the behavioral response to a drug like amphetamine on fixed-interval responding [58].
The history of reinforcing short IRTs before introducing the low-rate percentile schedule may be one reason that this study and an earlier one [49] were able to detect a MeHg effect while studies that did not establish high-rate responding before introducing the DRL also did not detect a MeHg effect [62,63]. The sensitivity of the present study may also be due to the use of a low-rate percentile schedule, which did not have a preponderance of short IRTs often seen with DRL responses. Of course, other differences among the studies could be important, too, including age of exposure and dosing regimen. If the history is important, however, then this suggests that the sensitivity of low-rate schedules in detecting deficits in response inhibition can be enhanced by first establishing a pattern of high-rate responding. Such an approach could assist with interpretation, too, since the presence of such a prepotent response is important when linking response inhibition to the executive functions [50]. This explanation could also apply to the spatial and discrimination reversals since the response that it reinforced during the original discrimination could be viewed as intruding into the first reversal.
The perseveration of high-rate responding as seen here can result in apparently paradoxical results. In two studies of gestationally exposed animals, MeHg enhanced acquisition of high rate responding during the rapid acquisition of fixed-ratio responding [49,64]. At very high ratios (75 responses required for a reinforcer) the exposed animals persisted in responding at very high rates even as unexposed controls showed poor responding with much pausin. Moreover, as noted earlier, when the schedule was abruptly changed to a differential reinforcement of low-rate (DRL) schedule, the MeHg-exposed rats persisted in a pattern of high response rates longer than control rats, although they did eventually acquire DRL responding [49]. In a study of gestational exposure to cadmium, an exposure that resulted in cortical damage, a similar effect was noted: exposed rats acquired large fixed-ratio schedule performance more rapidly than unexposed rats [65].
Methodologically, the low-rate percentile schedule provides an interesting contrast with the DRL schedule as an approach to studying the behavioral effects of drug or toxicant effects. Both are tactics that can be used to generate low response rates, but the DRL schedule imposes an unchanging criterion IRT and failure to meet the schedule requirement results in a decreased rate of reinforcement. In contrast, the percentile schedule used here imposes a titrating criterion that varies as a direct result of responding, making reinforcement more easily attainable and stabilizing the reinforcement rate. The stability in reinforcement rate was further assured further by placing criterion IRTs under a Random Interval 30″ schedule, so overall reinforcement rate remained constant for both the high-and low-rate percentile schedules. In the present study, the reinforcement schedule worked as intended, maintaining consistent reinforcement rates across a wide range of response rates. Thus, the percentile schedule placed under an overall Random Interval schedule is a useful tool because it does not impose an artificial ceiling or floor on response rates and it avoids interpretative complications due to changes in reinforcement rate and as a result may facilitate detecting effects that changing reinforcement rates could obscure.
During the low-rate challenge, responding under the unchanged DRH component was unaffected in any group, suggesting that the exposure-dependent effects on the median percentile IRT cannot be accounted for by aging or MeHg-related motor dysfunction. Since the DRH and percentile components were distinguished by specific stimuli, it appears that gestational MeHg exposure did not affect the discrimination between the two components even as the response rates under the low-rate percentile schedule decreased. This is consistent with earlier observations that low-level exposure does not affect discrimination learning [6,15]. For example, even in rats for which reversal learning was impaired, the original acquisition of a spatial discrimination proceeded similarly for control and MeHg-exposed rats [12,13]. Moreover, when rats performing a spatial discrimination were abruptly required to perform a visual discrimination, an exteroceptive shift [45,46], the transition seen by the MeHg-exposed rats were indistinguishable from control rats. This supports an earlier suggestion that low-level MeHg exposure does not affect discrimination learning [15].
The results here and in the other investigations discussed are generally consistent with neurochemical effects of gestational MeHg exposure. Enhanced sensitivity to dopamine agonists has been reported in young [66–68] and adult rats [20,38], which would be consistent with behavioral evidence of enhanced efficacy of primary reinforcers, since dopamine is closely linked to reinforcement processes [69,70] and especially to detecting changes in the source of reinforcement [71,72]. In addition, activity of the inhibitory neurotransmitter, GABA, is inhibited by gestational MeHg exposure [18,19]. Like the effects on dopamine pathways, the GABA effects are reversible and behaviorally relevant, since adult rats gestationally exposed show diminished sensitivity to the GABA agonist, pentobarbital [20]. The prolonged responding during extinction [12,13] or, here, after a transition from high- to low-rate responding is consistent with observations that drugs that promote GABA activity facilitate the extinction of operant behavior [73,74].
In a study of exposed children, prenatal exposure to low levels of MeHg (maternal hair averaged 0.5 ppm) produced substantial impairment of DRL schedule performance [75]. The acquisition of DRL performance was examined in 20 IRT bins through the course of a 1 h session. As in the present study, there were significant effects in the first encounter with the DRL schedule and some effects persisted to the last encounter, when responding was more or less at steady state. The extent to which prepotent responses may have been relevant in the Stewart et al. study is unclear, but often with children such responses may normally be present [50].
The absence of protection by selenium in the present study is in agreement with others that have reported no neuroprotection by selenium against MeHg’s developmental neurotoxicity. While both MeHg exposure and severe selenium have similar effects on thermoregulation, righting reflex, and locomotion in pre-weaning mice, selenium supplementation does not protection against these effects of MeHg when the two are both present [33]. Using physiological endpoints (e.g., body mass and mortality), Beyrouty was unable to find protection by selenium against the effects of developmental exposure to MeHg [35]. Using littermates of animals in the present study and identical postnatal exposure, Reed and colleagues found no protection be selenium on MeHg’s effects across several behavioral preparations, including discrimination reversals [13], elevated response rate during extinction [44], reinforcer efficacy [64], or sensitivity to cocaine [38]. The absence of protection seen with gestation exposure contrasts with the protection that is seen by selenium against adult-onset exposure. [9,29]. With adult-onset exposure, selenium might counteract oxidative damage caused by MeHg [76], bind MeHg and render it nontoxic [77], or by prevent selenium deficiency that might arise because MeHg–selenium binding reduces the amount of bioavailable selenium [34,78].
To summarize, gestational MeHg exposure impaired the acquisition of low-rate operant behavior after a history of high-rate responding. This can be viewed as akin to deficits in reversal learning that have been reported in previous studies, a failure of response inhibition, or both since these are not mutually exclusive accounts. There was no evidence of protection by selenium against MeHg’s developmental neurotoxicity.
Highlights.
Prenatal methylmercury (MeHg) exposure may impair development of the cortex.
Low-dose prenatal methylmercury exposure impaired low-rate lever-pressing in rats.
Impaired inhibitory control was linked to intrusion of high-rate response bursts.
Percentile schedules produce low response rates and adjust criteria dynamically.
Reinforcement rates were held constant over a wide range of response rates.
Footnotes
This article was supported by NIH ES 10865.
References
- 1.Landrigan PJ, Sonawane B, Butler RN, Trasande L, Callan R, Droller D. Early environmental origins of neurodegenerative disease in later life. Environmental Health Perspectives. 2005;113:1230–3. doi: 10.1289/ehp.7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amin-zaki L, Majeed MA, Clarkson TW, Greenwood MR. Methylmercury poisoning in Iraqi children: clinical observations over two years. British Medical Journal. 1978;1:613–6. doi: 10.1136/bmj.1.6113.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bakir F, Damluju SF, Amin-Zaki L, Murtadha M, Khaliki A, Al-Rawi NY, et al. Methyl mercury poisoning in Iraq. Science. 1973;181:230–41. doi: 10.1126/science.181.4096.230. [DOI] [PubMed] [Google Scholar]
- 4.Harada M. Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Critical Reviews in Toxicology. 1995;25:1–24. doi: 10.3109/10408449509089885. [DOI] [PubMed] [Google Scholar]
- 5.Barone S, Haykal-Coates N, Parran DK, Tilson HA. Gestational exposure to methylmercury alters the developmental pattern of trk-like immunoreactivity in the rat brain and results in cortical dysmorphology. Developmental Brain Research. 1998;109:13–31. doi: 10.1016/s0165-3806(98)00038-8. [DOI] [PubMed] [Google Scholar]
- 6.Rice DC. Sensory and cognitive effects of developmental methylmercury exposure in monkeys, and a comparison to effects in rodents. Neurotoxicology. 1996;17:139–54. [PubMed] [Google Scholar]
- 7.Rice DC. Evidence for delayed neurotoxicity produced by methylmercury. Neurotoxicology. 1996;17:583–96. [PubMed] [Google Scholar]
- 8.Newland MC. Methylmercury and fish nutrients in experimental models. In: Ceccatelli S, Aschner M, editors. Methylmercury and neurotoxicity (current topics in neurotoxicity) Springer; 2012. [Google Scholar]
- 9.Newland MC, Paletz EM, Reed MN. Methylmercury and nutrition. Adult effects of fetal exposure in experimental models. Neurotoxicology. 2008;29:783–801. doi: 10.1016/j.neuro.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newland MC, Yezhou S, Logdberg B, Berlin M. Prolonged behavioral effects of in utero exposure to lead or methyl mercury: reduced sensitivity to changes in reinforcement contingencies during behavioral transitions and in steady state. Toxicology and Applied Pharmacology (New York, NY) 1994;126:6–15. doi: 10.1006/taap.1994.1084. [DOI] [PubMed] [Google Scholar]
- 11.Newland MC, Reile PA, Langston JL. Gestational exposure to methylmercury retards choice in transition in aging rats. Neurotoxicology and Teratology. 2004;26:179–194. doi: 10.1016/j.ntt.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Paletz EM, Day JJ, Craig-Schmidt MC, Newland MC. Spatial and visual discrimination reversals in adult and geriatric rats exposed during gestation to methylmercury and n-3 polyunsaturated fatty acids. Neurotoxicology. 2007;28:707–19. doi: 10.1016/j.neuro.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reed MN, Paletz EM, Newland MC. Gestational exposure to methylmercury and selenium: effects on a spatial discrimination reversal in adulthood. Neurotoxicology. 2006;27:721–32. doi: 10.1016/j.neuro.2006.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilbert SG, Burbacher TM, Rice DC. Effects of in utero methylmercury exposure on a spatial delayed alternation task in monkeys. Toxicology and Applied Pharmacology (New York, NY) 1993;123:130–6. doi: 10.1006/taap.1993.1229. [DOI] [PubMed] [Google Scholar]
- 15.Newland MC, Paletz EM. Animal studies of methylmercury and PCBs: what do they tell us about expected effects in humans. Neurotoxicology. 2000;21:1003–27. [PubMed] [Google Scholar]
- 16.Shull RL, Gaynor ST, Grimes JA. Response rate viewed as engagement bouts: effects of relative reinforcement and schedule type. Journal of the Experimental Analysis of Behavior. 2001;75:247–74. doi: 10.1901/jeab.2001.75-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shull RL, Gaynor ST, Grimes JA. Response rate viewed as engagement bouts: resistance to extinction. Journal of the Experimental Analysis of Behavior. 2002;77:211–31. doi: 10.1901/jeab.2002.77-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Methylmercury Yuan Y. A potential environmental risk factor contributing to epileptogenesis. Neurotoxicology. 2012;33:119–26. doi: 10.1016/j.neuro.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O‘Kusky JR, McGeer EG. Methylmercury poisoning of the developing nervous system in the rat: decreased activity of glutamic acid decarboxylase in cerebral cortex and neostriatum. Brain Research (Amsterdam) 1985;353:299–306. doi: 10.1016/0165-3806(85)90219-6. [DOI] [PubMed] [Google Scholar]
- 20.Rasmussen EB, Newland MC. Developmental exposure to methylmercury alters behavioral sensitivity to d amphetamine and pentobarbital in adult rats. Neurotoxicology and Teratology. 2001;23:45–55. doi: 10.1016/s0892-0362(00)00112-4. [DOI] [PubMed] [Google Scholar]
- 21.Kuch DO, Platt JR. Reinforcement rate and interresponse time differentiation. Journal of the Experimental Analysis of Behavior. 1976;26:471–86. doi: 10.1901/jeab.1976.26-471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galbicka G, Platt JR. Interresponse-time punishment: a basis of shock-maintained behavior. Journal of the Experimental Analysis of Behavior. 1984;41:291–308. doi: 10.1901/jeab.1984.41-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galbicka G, Platt JR. Parametric manipulation of interresponse-time contingency independent of reinforcement rate. Journal of Experimental Psychology [Animal Behavior] 1986;12:371–80. [PubMed] [Google Scholar]
- 24.Newland MC, Rasmussen EB. Aging unmasks adverse effects of gestational exposure to methylmercury in rats. Neurotoxicology and Teratology. 2000;22:819–28. doi: 10.1016/s0892-0362(00)00107-0. [DOI] [PubMed] [Google Scholar]
- 25.Johnson JE, Bailey JM, Newland MC. Using pentobarbital to assess the sensitivity and independence of response-bout parameters in two mouse strains. Pharmacology, Biochemistry, and Behavior. 2011;97:470–8. doi: 10.1016/j.pbb.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Johnson JE, Pesek EF, Newland MC. High-rate operant behavior in two mouse strains: a response-bout analysis. Behavior Processes. 2009;81:309–15. doi: 10.1016/j.beproc.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Farina M, Frizzo ME, Soares FA, Schwalm FD, Dietrich MO, Zeni G, et al. Ebselen protects against methylmercury-induced inhibition of glutamate uptake by cortical slices from adult mice. Toxicology Letters (Amsterdam) 2003;144:351–7. doi: 10.1016/s0378-4274(03)00242-x. [DOI] [PubMed] [Google Scholar]
- 28.Ganther H, Goudie C, Sunde M, Kopecky M, Wagner P. Selenium: relation to decreased toxicity of methylmercury added to diets containing tuna. Science. 1972;175:1122–4. doi: 10.1126/science.175.4026.1122. [DOI] [PubMed] [Google Scholar]
- 29.Heath JC, Banna KM, Reed MN, Pesek EF, Cole N, Li J, et al. Dietary selenium protects against selected signs of methylmercury exposure and aging. Neurotoxicology. 2010;31:169–79. doi: 10.1016/j.neuro.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishikido N, Furuyashiki K, Naganuma A, Suzuki T, Imura H. Maternal selenium deficiency enhances the fetolethal toxicity of methyl mercury. Toxicology and Applied Pharmacology (New York, NY) 1987;88:322–238. doi: 10.1016/0041-008x(87)90207-9. [DOI] [PubMed] [Google Scholar]
- 31.Ralston NVC, Ralston CR, Blackwell JL, III, Raymond LJ. Dietary and tissue selenium in relation to methylmercury toxicity. Neurotoxicology. 2008;29:802–11. doi: 10.1016/j.neuro.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Schionning JD, Eide R, Ernst E, Danscher G, Moller-Madsen B. The effect of selenium on the localization of autometallographic mercury in dorsal root ganglia of rats. The Histochemical Journal. 1997;29:183–91. doi: 10.1023/a:1026493607861. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe C, Yin K, Kasanuma Y, Satoh H. In utero exposure to methylmercury and Se deficiency converge on the neurobehavioral outcome in mice. Neurotoxicology and Teratology. 1999;21:83–8. doi: 10.1016/s0892-0362(98)00036-1. [DOI] [PubMed] [Google Scholar]
- 34.Watanabe C, Yoshida K, Kasanuma Y, Kun Y, Satoh H. In utero methylmercury exposure differentially affects the activities of selenoenzymes in the fetal mouse brain. Environmental Research. 1999;80:208–14. doi: 10.1006/enrs.1998.3889. [DOI] [PubMed] [Google Scholar]
- 35.Beyrouty P, Chan HM. Co-consumption of selenium and vitamin E altered the reproductive and developmental toxicity of methylmercury in rats. Neurotoxicology and Teratology. 2006;28:49–58. doi: 10.1016/j.ntt.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 36.Berry MJ, Ralston NVC. Mercury toxicity and the mitigating role of selenium. EcoHealth. 2008;5:456–9. doi: 10.1007/s10393-008-0204-y. [DOI] [PubMed] [Google Scholar]
- 37.Ralston NVC, Raymond LJ. Dietary selenium’s protective effects against methylmercury toxicity. Toxicology (Limerick) 2010;278:112–23. doi: 10.1016/j.tox.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 38.Reed MN, Newland MC. Gestational methylmercury exposure selectively increases the sensitivity of operant behavior to cocaine. Behavioral Neuroscience. 2009;123:408–17. doi: 10.1037/a0014595. [DOI] [PubMed] [Google Scholar]
- 39.Newland MC, Paletz EM, Reed MN. Lactational exposure to mercury in experimental models. Neurotoxicology. 2009;30:160–3. [Google Scholar]
- 40.Newland MC, Reile PA. Blood and brain mercury levels after chronic gestational exposure to methylmercury in rats. Toxicological Sciences (Orlando, FL) 1999;50:106–16. doi: 10.1093/toxsci/50.1.106. [DOI] [PubMed] [Google Scholar]
- 41.Newland MC, Reed MN, LeBlanc A, Donlin WD. Brain and blood mercury and selenium after chronic and developmental exposure to methylmercury. Neurotoxicology. 2006;27:710–20. doi: 10.1016/j.neuro.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 42.R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. [Google Scholar]
- 43.Brackney RJ, Cheung TH, Neisewander JL, Sanabria F. The isolation of motivational, motoric, and schedule effects on operant performance: a modeling approach. Journal of the Experimental Analysis of Behavior. 2011;96:17–38. doi: 10.1901/jeab.2011.96-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reed MN, Newland MC. Prenatal methylmercury exposure increases responding under clocked and unclocked fixed interval schedules of reinforcement. Neurotoxicology and Teratology. 2007;29:492–502. doi: 10.1016/j.ntt.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 45.Chudasama Y, Robbins TW. Functions of frontostriatal systems in cognition: comparative neuropsychopharmacological studies in rats, monkeys and humans. Biological Psychology. 2006;73:19–38. doi: 10.1016/j.biopsycho.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 46.Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neuroscience and Biobehavioral Reviews. 2004;28:771–84. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 47.Laties VG, Weiss B, Clark RL, Reynolds MD. Overt mediating’ behavior during temporally spaced responding. Journal of the Experimental Analysis of Behavior. 1965;8:107–16. doi: 10.1901/jeab.1965.8-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Monterosso J, Ainslie G. Beyond discounting: possible experimental models of impulse control. Psychopharmacology (Berlin) 1999;146:339–47. doi: 10.1007/pl00005480. [DOI] [PubMed] [Google Scholar]
- 49.Paletz EM, Craig-Schmidt MC, Newland MC. Gestational exposure to methylmercury and n-3 fatty acids: effects on high- and low-rate operant behavior in adulthood. Neurotoxicology and Teratology. 2006;28:59–73. doi: 10.1016/j.ntt.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 50.Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychological Bulletin. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- 51.Paule MG, Green L, Myerson J, Alvarado M, Bachevalier J, Schneider JS, et al. Behavioral toxicology of cognition: extrapolation from experimental animal models to humans: behavioral toxicology symposium overview. Neurotoxicology and Teratology. 2012;34:263–73. doi: 10.1016/j.ntt.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richards JB, Sabol KE, Seiden LS. DRL interresponse-time distributions: quantification by peak deviation analysis. Journal of the Experimental Analysis of Behavior. 1993;60:361–85. doi: 10.1901/jeab.1993.60-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shull RL. Mathematical description of operant behavior: an introduction. In: Iversen IH, Lattal KA, editors. Experimental analysis of behavior. Parts 1 & 2: techniques in the behavioral and neural sciences. Amsterdam: Elsevier; 1991. pp. 243–82. [Google Scholar]
- 54.Weiss B, Laties VG, Siegel L, Goldstein D. A computer analysis of serial interactions in spaced responding. Journal of the Experimental Analysis of Behavior. 1966;9:619–26. doi: 10.1901/jeab.1966.9-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cole MR. The long-term effect of high- and low-rate responding histories on fixed-interval responding in rats. Journal of the Experimental Analysis of Behavior. 2001;75:43–54. doi: 10.1901/jeab.2001.75-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.LeFrancois JR, Metzger B. Low-response-rate conditioning history and fixed-interval responding in rats. Journal of the Experimental Analysis of Behavior. 1993;59:543–9. doi: 10.1901/jeab.1993.59-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tatham TA, Wanchisen BA. Behavioral history: a definition and some common findings from two areas of research. Behavior Analysis. 1998;21:241–51. doi: 10.1007/BF03391966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Urbain C, Poling A, Millam J, Thompson T. d-Amphetamine and fixed-interval performance: effects of operant history. Journal of the Experimental Analysis of Behavior. 1978;29:385–92. doi: 10.1901/jeab.1978.29-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hirai M, Okouchi H, Matsumoto A, Lattal KA. Some determinants of remote behavioral history effects in humans. Journal of the Experimental Analysis of Behavior. 2011;96:387–415. doi: 10.1901/jeab.2011.96-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weiner H. Controlling human fixed-interval performance. Journal of the Experimental Analysis of Behavior. 1969;12:349–73. doi: 10.1901/jeab.1969.12-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pattij T, Broersen LM, Peter S, Olivier B. Impulsive-like behavior in differential-reinforcement-of-low-rate 36 s responding in mice depends on training history. Neuroscience Letters. 2004;354:169–71. doi: 10.1016/j.neulet.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 62.Eccles CU, Annau Z, Eccles CU, Annau Z. Prenatal methyl mercury exposure: II. Alterations in learning and psychotropic drug sensitivity in adult offspring. Neurobehavioral Toxicology and Teratology. 1982;4:377–82. [PubMed] [Google Scholar]
- 63.Sable HJK, Eubig PA, Powers BE, Wang VC, Schantz SL. Developmental exposure to PCBs and/or MeHg: effects on a differential reinforcement of low rates (DRL) operant task before and after amphetamine drug challenge. Neurotoxicology and Teratology. 2009;31:149–58. doi: 10.1016/j.ntt.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Reed MN, Banna KM, Donlin WD, Newland MC. Effects of gestational exposure to methylmercury and dietary selenium on reinforcement efficacy in adulthood. Neurotoxicology and Teratology. 2008;30:29–37. doi: 10.1016/j.ntt.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Newland MC, Ng WW, Baggs RB, Gentry GD, Weiss B, Miller RK. Operant behavior in transition reflects neonatal exposure to cadmium. Teratology. 1986;34:231–42. doi: 10.1002/tera.1420340302. [DOI] [PubMed] [Google Scholar]
- 66.Hughes JA, Sparber SB. d-Amphetamine unmasks postnatal consequences of exposure to methylmercury in utero: methods for studying behavioral teratogenesis. Pharmacology, Biochemistry, and Behavior. 1978;8:365–75. doi: 10.1016/0091-3057(78)90072-2. [DOI] [PubMed] [Google Scholar]
- 67.Cagiano R, de Salvia MA, Renna G, Tortella E, Braghiroli D, Parenti C, et al. Evidence that exposure to methyl mercury during gestation induces behavioral and neurochemical changes in offspring of rats. Neurotoxicology and Teratology. 1990;12:23–8. doi: 10.1016/0892-0362(90)90108-o. [DOI] [PubMed] [Google Scholar]
- 68.Rossi AD, Ahlbom E, Ogren SO, Nicotera P, Ceccatelli S. Prenatal exposure to methylmercury alters locomotor activity of male but not female rats. Experimental Brain Research. 1997;117:428–36. doi: 10.1007/s002210050237. [DOI] [PubMed] [Google Scholar]
- 69.Wise RA. Dopamine, learning and motivation. Nature Review of Neurobiology. 2004;5:483–94. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 70.Spanagel R, Weiss F. The dopamine hypothesis of reward: past and current status. Trends in Neurosciences. 1999;22:521–7. doi: 10.1016/s0166-2236(99)01447-2. [DOI] [PubMed] [Google Scholar]
- 71.Schultz W, Tremblay L, Hollerman JR. Changes in behavior-related neuronal activity in the straitum during learning. Trends in Neurosciences. 2003;26:321–8. doi: 10.1016/S0166-2236(03)00122-X. [DOI] [PubMed] [Google Scholar]
- 72.Schultz W, Tremblay L, Hollerman JR. Reward prediction in primate basal ganglia and frontal cortex. Neuropharmacology. 1998;37:421–9. doi: 10.1016/s0028-3908(98)00071-9. [review, 30 refs] [DOI] [PubMed] [Google Scholar]
- 73.Leslie JC, Shaw D, Gregg G, McCormick N, Reynolds DS, Dawson GR. Effects of reinforcement schedule on facilitation of operant extinction by chlordiazepoxide. Journal of the Experimental Analysis of Behavior. 2005;84:327–38. doi: 10.1901/jeab.2005.71-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shaw D, Dawson GR, Reynolds DS, McCabe C, Leslie JC. Effects of chlordiazepoxide on extinction and re-acquisition of operant behaviour in mice. Behavioural Pharmacology (Oxford) 2004;15:225–32. [PubMed] [Google Scholar]
- 75.Stewart PW, Sargent DM, Reihman J, Gump BB, Lonky E, Darvill T, et al. Response inhibition during differential reinforcement of low rates (DRL) schedules may be sensitive to low-level polychlorinated biphenyl, methylmercury, and lead exposure in children. Environmental Health Perspectives (Research Triangle Park, NC) 2006;114:1923–9. doi: 10.1289/ehp.9216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ganther HE. Modification of methylmercury toxicity and metabolism by selenium and vitamin E: possible mechanisms. Environmental Health Perspectives (Research Triangle Park, NC) 1978;25:71–6. doi: 10.1289/ehp.782571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Møller-Madsen B, Danscher G. Localization of mercury in CNS of the rat. IV. The effect of selenium on orally administered organic and inorganic mercury. Toxicology and Applied Pharmacology (New York, NY) 1991;108:457–73. doi: 10.1016/0041-008x(91)90092-s. [DOI] [PubMed] [Google Scholar]
- 78.Ralston NVC. Selenium health benefit values as a seafood safety criteria. Eco-Health. 2008;5:442–55. doi: 10.1007/s10393-008-0202-0. [DOI] [PubMed] [Google Scholar]