Figure 10.
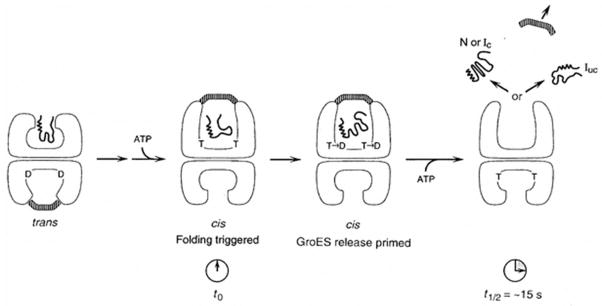
Schematic representation of the action of ATP in cis (top) and trans (bottom) compartments of the GroEL/ES complex during GroEL-GroES-mediated protein folding. The substrate polypeptide is initially bound in the trans compartment (bottom) of a GroEL-GroES complex, and a step of ATP-triggered substrate protein/GroES release then occurs. This results in formation of a cis complex (top) in which a second substrate polypeptide is sequestered in the GroEL central channel underneath GroES. The act of formation of the cis complex, through binding of ATP and GroES to the ring with bound polypeptide, triggers rapid release of the substrate polypeptide from the apical binding sites into the central channel, and folding begins (t0). Hydrolysis of ATP (T) to ADP (D) in the cis ring weakens the high affinity interaction between GroES and (cis)ATP-bound GroEL. Binding of ATP in trans subsequently causes release of GroES and polypeptide from the cis chamber. N, native; L, non-native conformation committed to completing folding to the native form (no longer recognizable by chaperonin); tUC, non-native form that must be rebound by chaperonin or by a different chaperone to reach its native form [Figure adapted with permission from an article by Rye et al., 1997].
