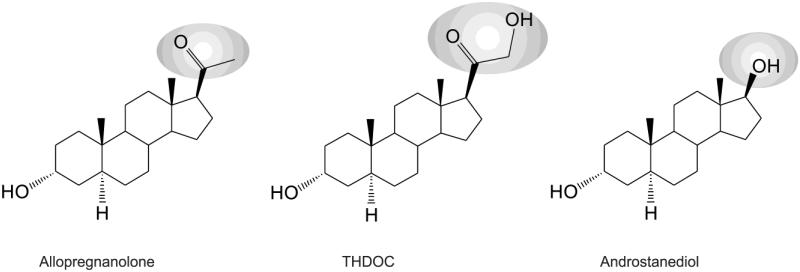
Fig. 1. Chemical structures of three prototype endogenous neurosteroids.
THDOC differs from allopregnanolone by a 21β-hydroxyl group, while androstane differs from allopregnanolone by a 17β-hydroxyl group instead of 17β-methyl-carbonyl group. Synthetic analogs of neurosteroids are prepared by additional moieties at C3-position (ganaxolone), C11-position (alphaxolone), and C2- and C11-positions (minaxolone).