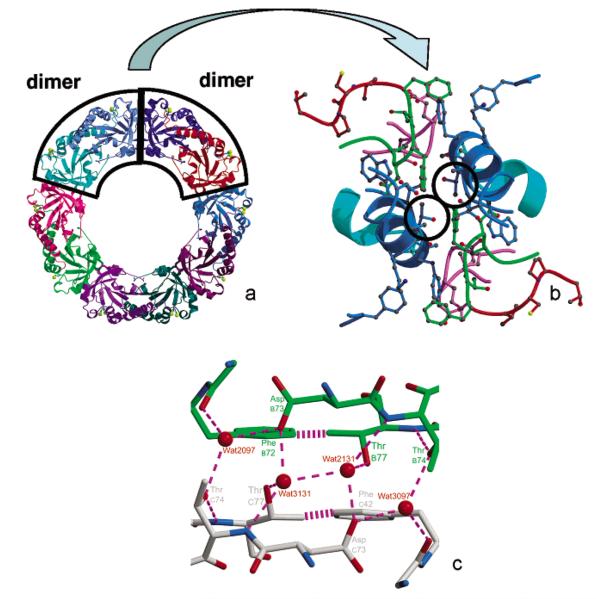
Figure 2.
Three views emphasizing the location of proximal Thr77 residues at the AhpC decamer-building interface. The dimer–dimer interface (panels a and b) in decameric AhpC (also known as the “alternate” or “A” interface) includes Thr residues in the opposing subunits (Thr77 and Thr77′) which are near one another, suggesting a potentially useful target for mutagenesis to destabilize decamers. In panel c, the close-up of this interface region shows that Oγ of Thr77 is proximal to Cγ of Thr77′ (3.8 Å) and to Oδ of Asp73′ (3.7 Å), across the dimer–dimer interface. Chain C (gray carbons) and chain B (green carbons) are colored according to atom type (red oxygens and blue nitrogens). Dashed lines represent hydrogen bonds (2.4–3.2 Å), and the striped lines represent the van der Waals interaction between Cγ2 of Thr77 and C∊1 of Phe42 (3.8 Å). An approximate 2-fold axis relates the two molecules at this interface and also relates the preferred hydration sites (water 2131/3131, and water 2097/3097, 2xxx and 3xxx designation for subunits b and c, respectively).