Figure 3.
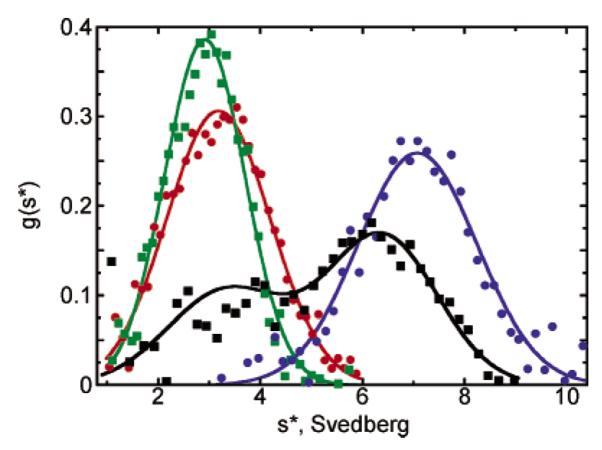
Sedimentation velocity studies of oxidized wild-type and Thr77 mutant AhpC proteins. Analytical ultracentrifugation studies of AhpC proteins were carried out with centrifugation at 42 000 rpm, 20 °C, and neutral pH. Eight to 16 consecutive 280 nm data sets for each run, where the boundaries had moved to approximately the middle of the cells, were included in the analyses by DCDT+ software to give the g*(s) distributions that are shown. Only data for the oxidized proteins (40 of at least 240 data points used in the fit) are shown. Curves fit to a single-species model (except for wild-type AhpC, which was fit to a two-species model) represent data for wild-type AhpC at 10 μM (black), T77V at 10 μM (blue), T77D at 100 μM (red), and T77I at 100 μM (green). All plots were normalized by area to ease comparisons. Results show that the T77V mutation actually promotes decamerization of the oxidized enzyme, whereas the T77I and T77D mutations destabilize the decameric forms of both the oxidized and reduced proteins (latter not shown).
