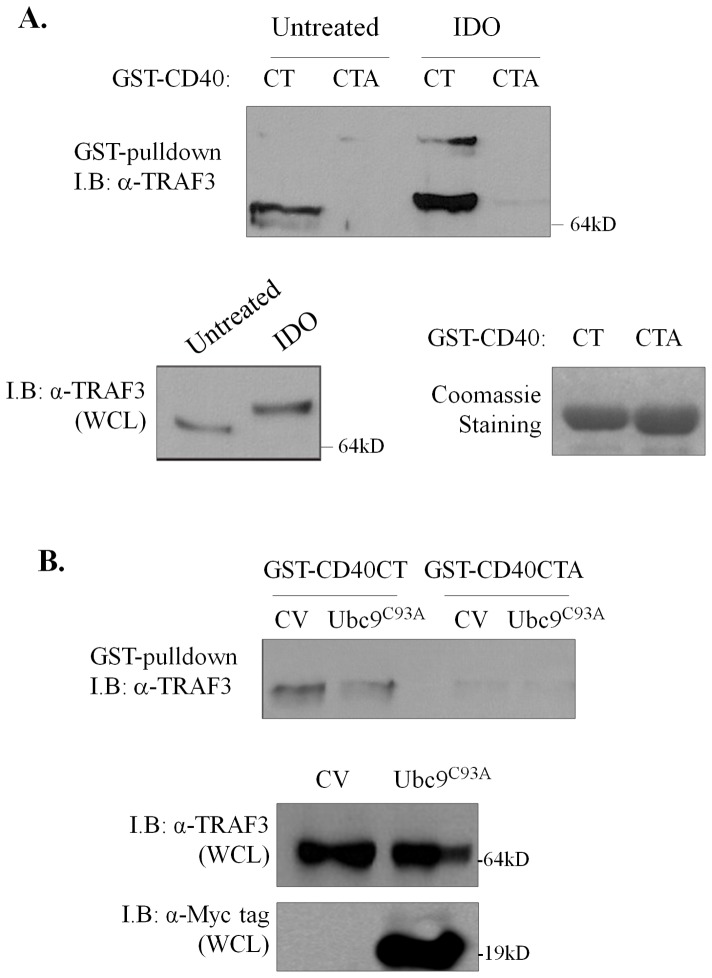
Figure 6. TRAF3 SUMOylation affects its CD40-interacting capacity.
(A) The binding of TRAF3 to GST-CD40CT increases when SUMO modification is maintained. HEK293 cells were lysed in the presence or absence of iodoacetamide (IDO) and lysates were incubated with bacterially produced GST-CD40 C-terminus (CT) or, as control GST-CD40CTA, carrying a T254→A mutation (GST-CD40CTA) which abolishes interaction with TRAF3, bound to glutathione sepharose beads. Interacting proteins were fractionated by SDS-PAGE and immunoblotted (I.B.) with anti-TRAF3 Ab. Whole cell lysates (WCL; 30 µg) were analyzed by immunoblot for TRAF3 expression levels. Lower right panel: Coomassie-stained gel showing GST-CD40CT and GST-CD40CTA produced in bacteria. (B) Over-expression of “dominant-negative” Ubc9C93A reduces binding of TRAF3 to CD40. HEK293 cells were transfected with Ubc9C93A or control vector (CV), lysates were obtained using an IDO-containing lysis buffer and incubated with GST-CD40CT or GST-CD40CTA bound to glutathione sepharose beads. Interacting proteins were fractionated by SDS-PAGE and immunoblotted (I.B.) with anti-TRAF3. Whole cell lysates (WCL; 30 µg) were analyzed for TRAF3 and Ubc9C93A expression levels by immunoblotting using anti-TRAF3 and Myc tag Abs, respectively. Results in (A) & (B) are representative of 3 independent experiments.