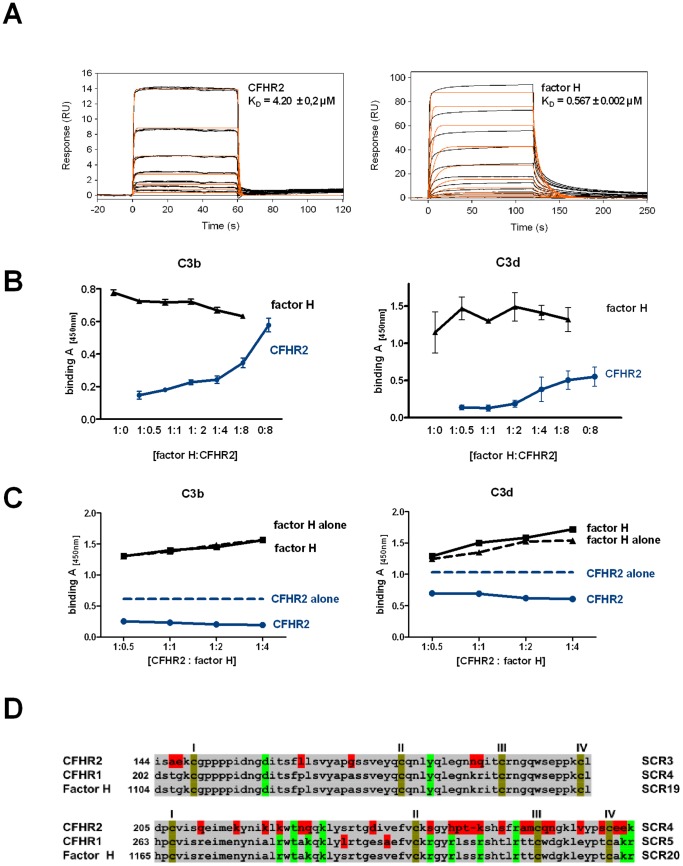
Figure 3. CFHR2 binds to C3b and C3d and does not compete off factor H.
(A) Real-time in vitro SPR binding analysis of CFHR2 (left panel) and factor H (right panel) to sensor immobilized C3b. CFHR2 and factor H binding sensograms (black lines) are shown overlaid with the best fit derived from a 1∶1 interaction model including a mass transport term (red lines). As CFHR2 forms exclusively dimers, one CFHR2 dimer was regarded as one molecule. (B) CFHR2 slightly competes off factor H from binding to C3b (left panel) and does not effect factor H binding to C3d (right panel). Mean values ± SD of three independent experiments and the molar ratios of factor H (1 = 66 nM) to CFHR2 are shown. (D) factor H binds preferentially to C3b and to C3d in the presence of constant amounts of CFHR2 (1 = 66 nM). Constant amounts of CFHR2 and increasing concentrations of factor H were incubated with immobilized C3b or C3d. Binding rates of CFHR2 and factor H alone to C3b or C3d are represented by dashed lines. A representative experiment is shown. (E) C-terminal amino acid sequence alignment of CFHR2 SCR3 and SCR4 with factor H SCR19 and SCR20. Conserved cysteins (I–IV, brown), identical (grey) and non-identical amino acids (red) are marked. Charged amino acids that are relevant for C3b and heparin binding are indicated (green).