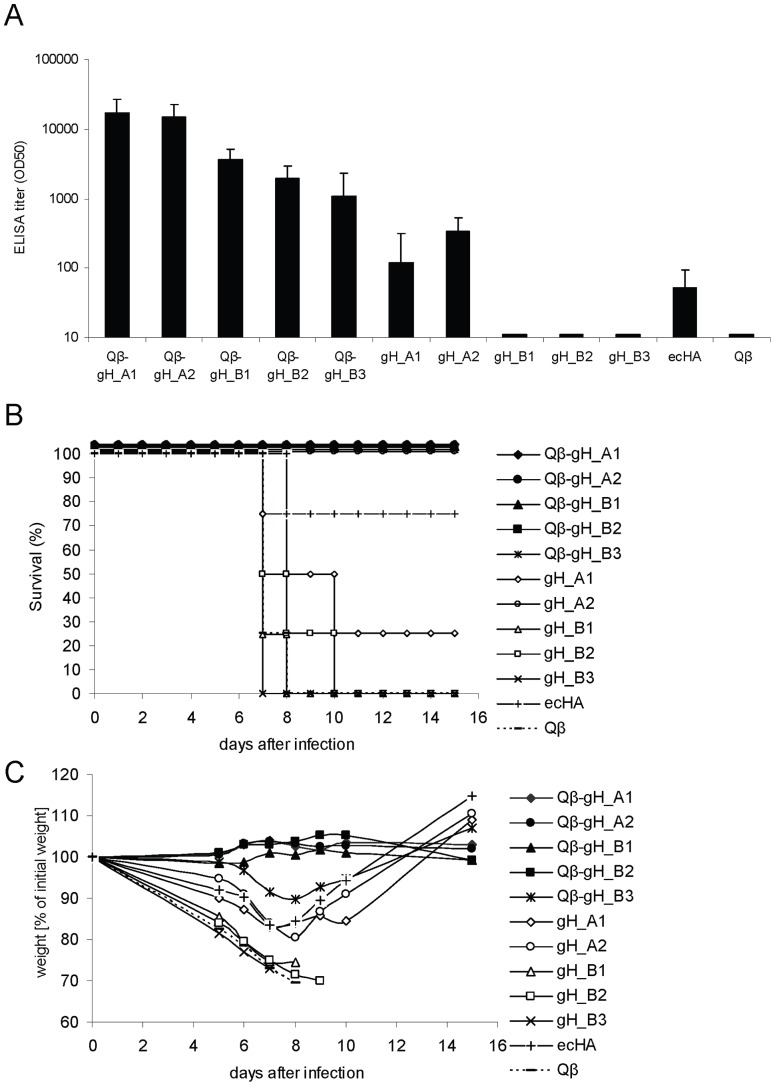
Figure 2. Immunogenicity and in vivo efficacy of Qβ -gH vaccines of influenza A/PR/8/34.
A Groups of six-weeks old female BALB/c mice (n = 4) were immunized subcutaneously once with 15 μg of Qβ -gH conjugate vaccines, 15 μg gH proteins alone, 15 μg full-length trimeric ectodomain of HA or 15 μg Qβ in the absence of adjuvants. IgG antibody titers specific for native HA protein of A/PR/8/34 were measured at day 21 after immunization. ELISA titers are indicated as IgG titer at which half-maximal OD was reached (OD50). B,C On day 27, mice were challenged i.n. with 10× LD50 of influenza virus A/PR/8/34. Survival B and weight loss C of individual mice were monitored for 16 days post challenge. The following survival rates were achieved: Qβ -gH_A1 (100%), Qβ -gH_A2 (100%), Qβ -gH_B1 (100%), Qβ -gH_B2 (100%), Qβ -gH_B3 (100%), gH_A1 (25%), gH_A2 (100%), gH_B1 (0%), gH_B2 (0%), gH_B3 (0%), ecHA (75%), Qβ (0%).