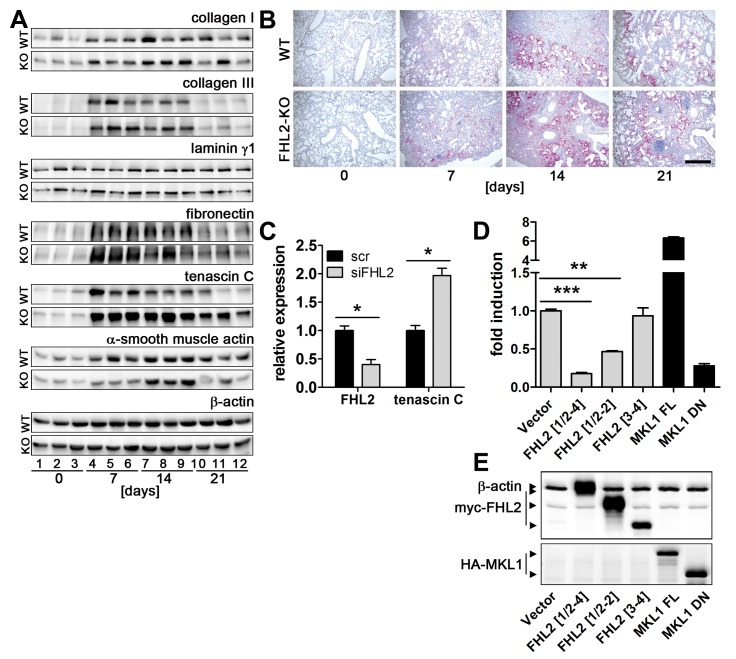
Figure 2. FHL2 inhibits expression of tenascin C.
(A) FHL2 WT and knockout mice were administered BLM for the indicated times. The left lung was then lysed with RIPA buffer and 20 µg of total lysates were analysed by Western blotting for the expression of different proteins. N = 2, n = 5 to 6 per time point, see Figure S3 for details. Lung lysates from three animals per time point are shown. Equal protein loads were verified by β-actin immunoblotting. (B) Paraffin lung sections of control and BLM-induced mice were stained for tenascin C (red). Nuclei (blue) were counterstained with haematoxylin. Bar=500 µm. (C) Immortalised embryonal fibroblasts from C57Bl/6 mice were transfected with scrambled or FHL2-specific siRNA for 24 h and the expression of tenascin C examined by TaqMan qRT-PCR. N = 3. (D) HEK 293 cells were cotransfected for 24 h with a luciferase reporter gene construct containing a 2000-bp tenascin C promoter sequence and indicated plasmids and the luciferase activity was then measured. Relative scores are presented. Mean values of N = 4 shown. (E) The expression of transfected plasmids was verified by Western blotting. A mixture of anti-myc and anti-actin antibodies was used to visualize FHL2 protein and the loading control β-actin on the same blot (upper panel). FHL2[1/2-4] represents the full length protein containing aa 1-279. FHL2[1/2-2] and FHL2 [3,4] represent C-terminal and N-terminal truncations containing the LIM domains ½ to 2 and LIM domains 3 to 4 or aa 1-157 and aa 159-279, respectively, and are described in [28]. MKL1 FL represent the full-length MKL1 protein and MKL1 DN, the dominant-negative MKL1 protein truncated at its N-terminal PPEL motifs and C-terminal transactivation domain, described in [29]. The MKL1 proteins were used as positive and negative control, respectively, for TNC promoter activity.