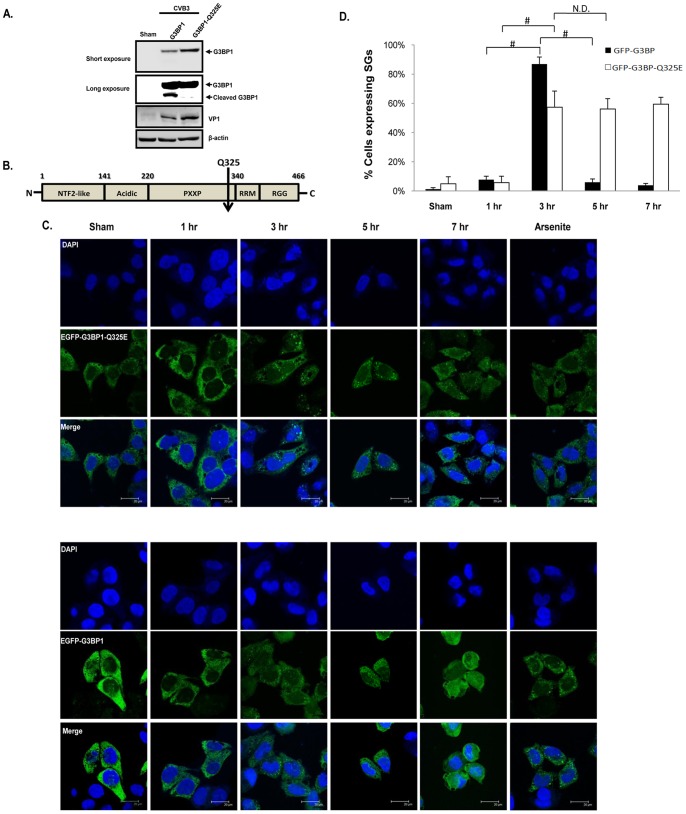
Figure 6. G3BP1 is cleaved at amino acid Q325 and a cleavage-resistant G3BP1 mutant restores SG formation at ∼5 hrs post-infection.
(A) HeLa cells were transfected with FLAG-G3BP1 or FLAG-G3BP1Q325E mutant for 48 hrs, followed by CVB3 infection at an MOI of 10 for 7 hrs. Western blotting was performed to examine G3BP1 cleavage using an anti-FLAG antibody. Protein expression of VP1 and β-actin was examined as an infection and loading control, respectively. (B) Schematic diagram of full length G3BP1 and the cleavage site. The arrow indicates the cleavage point at amino acid Q325 of G3BP1. NTF2-like, Nuclear Transport Factor 2-like; PXXP, SH3-domain binding domain of Ras-GAP; RRM, RNA Recognition Motif; RGG, Arginine-Glycine-rich region. (C) HeLa cells stably expressing GFP-G3BP1Q325E (upper panels) or GFP-G3BP1 (lower panels) were sham- or CVB3-infected at an MOI of 10 for different times as indicated. Intracellular distribution of G3BP1 was examined using confocal microscopy. Cell nuclei were counterstained with DAPI. Cells treated with 50 mM arsenite for 1 hr were used as positive controls. (D) Quantitation of G3BP1-SG formation from (C). Results are presented as mean ± SD (n = 10 images), #p<0.001, N.D., No statistical difference.