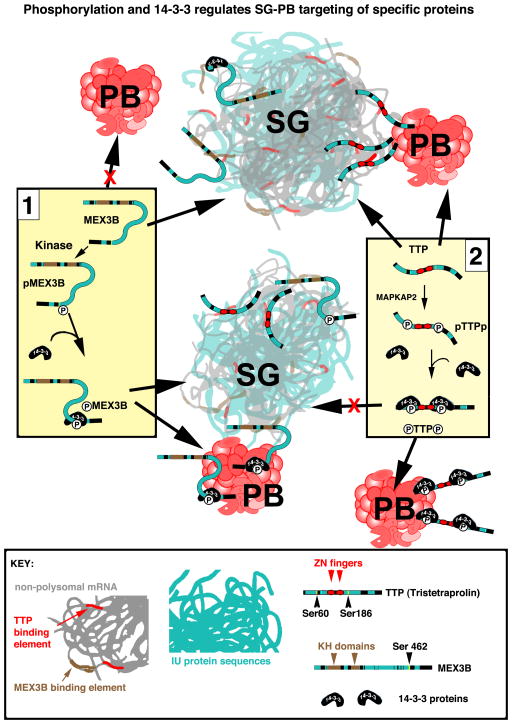
Fig. 2. Phosphorylation and 14-3-3 regulates SG-PB targeting of specific proteins.
MEX3B (Panel 1) possesses two KH domains (brown) and considerable ID sequence (aqua, also see Fig. 3). Non-phosphorylated MEX3B associates with SGs but not PBs. Phospho-MEX3B associates with both SGs and promotes SG-PB fusion 36. Its interaction with PBs requires phosphorylation of serine 462 and 14-3-3 binding, which are not required for its targeting to SGs 36. In contrast (Panel 2), non-phosphorylated TTP binds mRNA through its zinc fingers (red), associates with both SGs and PBs, and tethers SG and PB together 32, 34. MAPKAP2-induced phosphorylation of serines 60 and 186 promotes 14-3-3 binding, removing TTP from SGs while allowing its continued association with PBs 34, leading to the separation of SGs from PBs. The binding of 14-3-3 proteins to ID regions (proteins shown to scale) may stabilize the disordered regions, “freezing” them into a locked conformation (represented here by a rigid linear shape), thus taking TTP and perhaps other individual SG-associated proteins “out of phase” with the rapid and fleeting interactions within SGs.