Abstract
The topipotency of the germline is the full manifestation of the pluri- and multipotency of embryonic and adult stem cells, thus the germline and stem cells must share common mechanisms that guarantee their multipotentials in development. One of the few such known shared mechanisms is represented by Piwi proteins, which constitute one of the two subfamilies of the Argonaute protein family. Piwi proteins bind to Piwi-interacting RNAs (piRNAs) that are generally 26–31 nucleotides in length. Both Piwi proteins and piRNAs are most abundantly expressed in the germline. Moreover, Piwi proteins are expressed broadly in certain types of somatic stem/progenitor cells and other somatic cells across animal phylogeny. Recent studies indicate that the Piwi-piRNA pathway mediates epigenetic programming and post-transcriptional regulation, which may be responsible for its function in germline specification, gametogenesis, stem cell maintenance, transposon silencing, and genome integrity in diverse organisms.
Keywords: Argonaute, Piwi, piRNA, transposon, epigenetic regulation, post-transcriptional regulation
Introduction
Stem cells possess the unique ability to undergo self-renewing divisions, in order to self-maintain and to provide a continual supply of differentiated cells for their resident tissues (67). Depending on how many types of differentiated daughter cells a specific type of stem cell can produce, stem cells are classified as pluripotent, multipotent or unipotent. Pluripotent stem cells, known to exist as mammalian embryonic stem cells, can give rise to all types of tissues in an individual, but not extra-embryonic tissues. Multipotent stem cells, such as hematopoietic stem cells, are able to give rise to many differentiated cell types. Germline stem cells, which give rise to a constant supply of gametes, are unipotent. However, germ cells have the intrinsic capability of producing a totipotent zygote that ultimately gives rise to all cell types. Therefore, although highly specialized, germ cells retain totipotency and likely use the same or similar mechanisms that regulate stem cells. Stem cells possess great therapeutic potentials in regenerative medicine. In order to effectively harness this power, extensive efforts have been invested in exploring regulatory mechanisms that underlie their maintenance and differentiation. These research efforts have revealed small RNA pathways as key regulators of diverse types of stem cells, which are best known to repress the translation of specific mRNAs in stem cells and differentiated daughter cells (35).
Small RNAs often regulate gene expression by guiding Argonuate (Ago) protein-containing complexes in a nucleotide sequence-specific manner to different sites for molecular actions (31). Ago proteins have a variable N-terminal domain followed by a highly conserved PAZ domain, which together with the Mid-domain binds a small RNA (111). The C-terminal domain, called the PIWI domain, resembles RNase H and is often capable of cleaving target RNAs (41, 83). The Ago protein family is divided into the Ago and Piwi subfamilies based on phylogenetic analysis. Ago subfamily proteins bind microRNAs (miRNA) and small interfering RNAs (siRNA), which are both made from double-stranded precursors. In animal cells, precursors are provided either endogenously (miRNAs and endo-siRNAs) or exogenously (siRNAs), and both are ultimately processed by Dicer into 20–22 nucleotide small RNAs (49). Both miRNAs and siRNAs bind to Ago subfamily proteins, which are ubiquitously expressed in animal tissues to repress gene expression by promoting heterochromatin formation, mRNA turnover, and/or translational repression (37, 128). In contrast, Piwi protein expression is mostly restricted to germ cells and stem cells (116). Piwi proteins bind to piwi-interacting RNAs (piRNAs, generally 26–31 nucleotides in length), which are processed in a dicer-independent manner from long single-stranded precursors (3, 39, 44, 105). In this review we discuss the biological functions of Piwi proteins in animal germ cells and stem cells. Piwi proteins are required to maintain fertility and repress transposons in the germline and regulate gene expression at the epigenetic, post-transcriptional, and translational levels. Studies in animals from diverse taxa also demonstrate that Piwi proteins have a conserved stem cell function. Therefore, understanding how Piwi proteins regulate gene expression is key to understanding the regulation of both germline and stem cell identity, maintenance, and differentiation.
The expression and function of Piwi proteins in the germline
Piwi proteins are required for fertility
The expression of Piwi proteins is enriched in the germline of many animals. In Drosophila, there are three Piwi proteins: Piwi, Aubergine (Aub), and Argonaute3 (Ago3), all of which are expressed in the male and female germline (9, 22, 42, 65) (Table 1). In the Drosophila embryo Piwi and Aub proteins are enriched in the pole cells, which give rise to the germ cells (42, 75). In the adult ovary and testis, Piwi is enriched in the nucleus of both the germ and somatic cells of the gonads (23). Aub and Ago3, however, are selectively expressed in the germ cells of adult ovaries and are enriched in the cytoplasmic polar granules or germ plasm, which is presumably the precursor of the nuage, a structure common to all animal germ cells (9). In mouse, there are also three mouse Piwi homologs, Miwi (murine Piwi, Piwi-like protein 1), Mili (Piwi-like protein 2), and Miwi2 (Piwi-like protein 4) (Table 1), which display distinct expression patterns and functions during male germ cell specification and differentiation (13, 27, 57). Mili and Miwi proteins are mostly, if not exclusively, localized to the cytoplasm. Miwi expression is found in spermatocytes to elongating spermatids and Mili is expressed in spermatogonia to round spermatids (27, 117). Both proteins are enriched in nuage and its derivative, the chromatoid body in spermatids (57, 117, 124). Miwi2, which is both nuclear and localized to the perinuclear nuage, has a relatively narrow expression window during spermatogenesis, which is detected from 18 days post coitum (dpc) in the gonocytes to 3 days post partum (dpp) in spermatogonia stem cells (13). Two homologs of Piwi were identified in Zebrafish: Ziwi (Zebrafish Piwi), and Zili (Zebrafish Piwi-like) (43, 44) (Table 1). Ziwi is expressed in the nuage of early embryos and in both female and male germ cells of the adult fish, with the highest expression seen during the mitotic and early meiotic stages of germ cell differentiation (44). Zili expression is first detected in PGCs from 3 days post-fertilization (dpf) and after 21 dpf, Zili is found in cytoplasmic granules. In the adult, Zili is present throughout oogenesis and in both mitotic and meiotic male germ cells, but expression is not detected in mature sperm (43). The C. elegans genome encodes two Piwi orthologs: prg-1 and prg-2 (piwi-related gene) (Table 1). Prg-1, is expressed in peri-nuclear P-granules of the male germ cells (123). Therefore, the enrichment of Piwi protein in the nuage of germ cells is evolutionarily conserved.
Table 1.
Expression and function of Piwi proteins in animals.
| Expression* | Species | Name | Mutant phenotype | References |
|---|---|---|---|---|
| Germline | Drosophila melanogaster | Piwi | ♂♀ sterile | (22, 23) |
| Aubergine | ♂♀ sterile | (42, 107, 126) | ||
| Argonaute3 | ♀ sterile ♂ semifertile |
(65) | ||
| Mus musculus | Miwi (PiwiL1) | ♂ sterile | (27) | |
| Mili (PiwiL2) | ♂ sterile | (57) | ||
| Miwi2 (PiwiL4) | ♂ sterile | (13) | ||
| Danio rerio | Ziwi | ♂♀ sterile | (44) | |
| Zili | ♂♀ sterile | (43) | ||
| C. elegans | Prg-1 | sterile at 25°C | (123) | |
| Prg-2 | No obvious defect | (123) | ||
| Totipotent Stem Cells | Ephydatia fluviatilis (Sponge) | EfPiwiA | N/A | (34) |
| EfPiwiB | N/A | |||
| Tentacle Stem Cell | Clytia hemisphaerica (Hydrozoan) | ChePiwi | N/A | (28) |
| Somatic Stem Cells, Germ Cells | Pleurobrachia pileus (Ctenophore) | PpiPiwi1 | N/A | (2) |
| PpiPiwi2 | ||||
| Totipotent Stem Cells | Botrylloides leachi (Colonial Ascidian) | Bl-Piwi | Unabl to regenerate | (99) |
| Totipotent Stem Cells | Schmidtea mediterranea (Planria) | Smedwi-2 | Unable to regenerate | (85, 95) |
| Smedwi-3 | ||||
| Totipotent Stem Cells, Germ Cells | Macrostomum lignano (Marine Flat Worm) | MacPiwi | Loss of stem cells | (25) |
| Hematopoietic Stem Cells, Germ Cells | Homo sapiens | Hiwi | N/A | (94, 109) |
If germline expression is not listed this does not necessarily mean Piwi expression is absent from the germline, but rather that it was not experimentally tested.
Drosophila Piwi is required for the self-renewal of germline stem cells (GSCs). In piwi mutant flies, GSCs are depleted and the gonads contain only a few egg chambers or sperm bundles, which causes infertility in females and males, respectively (22, 68). In ago3 mutant male flies, GSCs are not properly maintained, either, causing reduced fertility (65). Miwi2 and Mili mutant mice show arrest of spermatogonia and the complete loss of male germ cells at three and six months, respectively, strongly suggesting a role for these proteins in GSC maintenance (13, 117). Finally, RNAi knockdown of both prg-1 and prg-2 in C. elegans leads to decreased germ cell proliferation (22). Therefore, Piwi proteins have a conserved role in maintaining GSCs during gametogenesis in animals.
In addition to maintenance of the GSCs, Piwi proteins are required for other aspects of gametogenesis. In mili mutants, spermatogenesis is blocked at the mid-pachytene stage; whereas in miwi mutant males, spermatogenesis is arrested at the early stages of spermiogenesis—a cellular morphogenic process through which round spermatids become mature sperm (27, 57). Similarly, in C. elegans, prg-1 mutant adults are sterile at 25°C due to defective spermatogenesis (123). In zebrafish ziwi and zili mutants, germ cells are lost in both males and females due to elevated levels of apoptosis at the various stages of germ cell development (43, 44), which might be an indirect consequence of arrested meiosis and failure in transposon silencing.
Piwi proteins are also involved in embryogenesis and germline specification. In Drosophila, Piwi proteins are required early in embryogenesis for patterning and pole cell formation. Both Piwi and Aub are essential for the formation of pole cells in Drosophila (42, 75). In addition, all three Drosophila Piwi proteins are required for proper body-axis patterning during early embryogenesis (22, 65, 107). Because Piwi proteins are required for formation of the germline and/or patterning of the embryo, techniques such as clonal analysis are required to further interrogate the functions of these proteins in the adult germline. From the above review, it is clear that Piwi proteins have diverse functions in gametogenesis, many of which are conserved in animals.
Piwi proteins repress transposon activation in the germline
Transposable elements (TE) are DNA elements that can transpose to new positions in the genome. Significant percentages of piRNAs in Drosophila, C. elegans, mice, and zebrafish are derived from intergenic regions containing TEs and repetitive sequences. Expectedly, Piwi proteins are required to repress transposons in the germline (1, 9, 44, 123). In Drosophila, approximately 75% of piRNAs are derived from repetitive sequences; Mutations in Piwi, Aub, and Ago3 result in transposon de-repression in the germline (54, 65, 118). In the mouse, loss of Mili, Miwi, and Miwi2 also leads to transposon de-silencing in the male germline (5, 13, 39, 125). In prenatal testis, Mili and Miwi2 associate with piRNAs enriched in transposon sequences (~47%) (4, 58). After birth, 35% of Mili-bound pre-pachytene piRNAs are derived from transposons (5). However, Miwi-bound adult pachytene piRNAs are not significantly enriched in transposon sequences (~15%), suggesting an additional function for Miwi in the mouse male germline (39, 125). In zebrafish and Xenopus, piRNAs are significantly enriched in transposon sequences suggesting a conserved function in transposon repression for Piwi proteins in vertebrates (43, 60).
Piwi proteins interact with Tudor-domain containing proteins (Tdrd’s) in the germline
To understand the molecular mechanisms of Piwi protein functions, many recent studies have focused on identifying and characterizing Piwi-interacting proteins. The N-termini of Piwi proteins contain conserved RG/RA motifs with dimethylated arginines that are required for Piwi binding to Tudor domains (119). Structural analysis of Tdrd2 (a.k.a. Tdrkh) and the ancestral staphylococcal nuclease domain-containing 1 (SND1) protein provides a binding mechanism of the Tudor domain to Piwi proteins (17, 69, 71). Piwi proteins contain multiple RG/RA repeats, which are potential methylation sites and may recruit different Tudor proteins. Proteins containing multiple Tudor domains potentially can bind to different Piwi proteins simultaneously to serve different molecular functions.
In the mouse, there are 28 Tudor family proteins (based on a search result from http://pfam.sanger.ac.uk). Several labs have demonstrated that different mouse Tudor proteins interact with Piwi proteins in various but specific combinations (17, 55, 97, 110, 119, 124) (Table 2). For example, Tdrd1 interacts with Mili, Miwi and Miwi2, but the Mili/Tdrd1 interaction is the strongest (17, 119, 124). Tdrd1, which contains four Tudor domains and one MYND domain, is essential for spermatogenesis (86). Similar to mouse Piwi proteins, Tdrd1 is predominantly localized to the nuage in spermatogonia and spermatocytes, as well as to a nuage-derived structure, the chromatoid body, in late spermatocytes and round spermatids (20). In the tdrd1 mutant, expression and localization of Mili is not affected, however, Miwi2 localization is disrupted with no change in expression levels (97, 110, 119), indicating that Trdr1 is specifically required for the proper localization of Miwi2. In the tdrd1 mutant mouse, piRNAs from exonic reads are overrepresented (48%) compared to wildtype (16%) (97). This suggests that Tdrd1 may perform quality control in the selection of certain transcripts to undergo piRNA biogenesis.
Table 2.
Interactions between Piwi protein and Tudor-domain containing protein in Drosophila and the mouse.
| species | Piwi protein | Tudor protein | References |
|---|---|---|---|
| Mus musculus | Miwi | Tdrd1/MTR1; Tdrd2/Tdrkh; Tdrd4/RNF17; Tdrd6; Tdrd7; Tdrd8; Tdrd9 | (17, 51, 119, 120) |
| Mili | Tdrd1/MTR1; Tdrd2/Tdrkh; Tdrd6 | (17, 55, 97, 110, 119, 124) | |
| Miwi2 | Tdrd1/MTR1; Tdrd2/Tdrkh; Tdrd9 | (55, 110, 119, 124) | |
| Drosophila melanogaster | Piwi | Papi; Yb | (72, 93, 104) |
| Aubergine | Tudor; Papi; Papi | (24, 50, 51, 72, 82) | |
| Argonaute 3 | Tudor; Papi | (72, 82) |
Tdrd9, which associates with Miw2, is required for meiosis during spermatogenesis. The loss-of-function mutation of tdrd9 results in spermatogenic arrest due to chromosome synapses failure (110). In fetal spermatogonia, Tdrd9 and Miwi2 co-localize in granules with the P-body markers DDX6 and GWB (6). Miwi2/Tdrd9 granules are distinct from Mili/Tdrd1 granules, which are finer and more numerous than Miwi2/Tdrd9 granules (6, 110). Tdrd9 is not required for the localization of Mili, Miwi, and Miwi2, and Miwi2 is not required for the localization of Tdrd9 and Tdrd1 (119). In Mili mutants, Tdrd9 nuage localization is lost and in Tdrd1 mutants both Tdrd9 and Miwi2 are dispersed in the cytoplasm, while Mili is not affected (110). Taken together, these data demonstrate the interdependence of Tudor family and Piwi family proteins and suggests that the Miwi2/Tdrd9 complex is a downstream of the Mili/Tdrd1 complex.
The interaction between Piwi and Tudor proteins also exists in Drosophila. The germline gene Tudor contains 11 Tudor-domain repeats and is required for polar granule formation (8, 91, 115). Aub and Ago3 bind to Tudor in a sDMA-dependent manner and this binding is required for germ plasm assembly and PGC specification (24, 50, 51, 82) (Table 2). In Drosophila, PRMT5 is required for arginine methylation of Piwi proteins (50, 72). Tudor appears to form various heteromeric complexes with Aub and AGO3, which are lost in prmt5 mutants (82). In the fly tudor mutant, the expression levels of Aub and Ago3 are not affected, but similar to mice, the identity of bound piRNAs is significantly changed (82). A novel Tudor containing protein in Drosophila, PAPI (Partner of Piwis), binds to the N-terminus of Ago3 in the nuage. Ago3 is miss-localized from nuage in papi or prmt5 mutants, while PAPI localization to nuage is not affected in ago3 or prmt5 mutant flies. This suggests that PAPI lies upstream of a pathway regulating Ago3 function (72).
Similar to the Piwi family, loss of Tudor proteins in Drosophila and mouse leads to transposon de-silencing. In mouse, loss of Tdrd1 and Tdrd9 leads to transposon overexpression and altered piRNA profiles (97, 110). However, in Tdrd6 mutant mice, transposon up-regulation is not observed (120). In Drosophila, Tudor domain containing proteins, such as Spindle E, Krimper, Tajas, PAPI, and Yb, function in silencing transposons (66, 88, 93, 104, 118). Therefore, Tudor proteins have a conserved role in the Piwi/piRNA pathway and silencing transposons in the germline.
The Piwi-piRNA complex mediates epigenetic function
Epigenetic mechanisms ensure heritable patterns of gene expression by regulating chromatin structure through histone modification and/or DNA methylation (113). Small RNAs are required for epigenetic processes, such as heterochromatin formation and maintenance by the RNA interference pathway. For example, in Schizosaccharomyces pombe, the RNAi machinery produces siRNAs from transcripts arising from centromeric repeat regions. Subsequently, the RNA Induced Transcriptional Silencing (RITS) complex, which contains Ago1 bound to these siRNAs, is targeted to nascent transcripts at the centromeres, leading to the formation and maintenance of heterochromatin via the recruitment of chromatin modifying and binding proteins. These proteins include the histone H3 lysine 9 (H3K9) methyltransferase, Clr4, and heterochromatin protein 1 (HP1, also called SWI6) (12, 121, 122).
Recent evidence suggests that the Piwi/piRNA complex may regulate epigenetic states via a similar mechanism in multicellular organisms. Drosophila Piwi protein is predominately located in the nucleus of both germline and somatic cells. Piwi proteins bind to chromatin, and its mutation suppresses position effect variegation (PEV) in somatic cells (10, 84, 129). In somatic cells, Piwi binds to polytene chromosomes in an RNA-dependent manner as well as directly binds to HP1a, a central player in heterochromatic gene silencing (10). In Piwi and Aub mutants, HP1 and HP2 are delocalized from polytene chromosomes and the levels of H3K9 methylation are reduced (84). Taken together, these studies suggest that Piwi acts upstream of HP1 and H3K9 methylation (Figure 1A) (10, 84). The HP1 homolog Rhino in Drosophila is required to silence transposons through a distinct unknown pathway in which Rhino functions upstream of Ago3 and Aub, but may have a less critical role in Piwi-dependent heterochromatin formation (53). In contrast to the silencing role of Piwi in certain regions of the genome, Piwi is required for transcriptional activation of the telomere-associated sequence of the right arm of chromosome 3 (3R-TAS) (129). Altogether, these studies show that Piwi can either activate or silence gene expression and this may be dependent upon the local chromatin environment and/or its co-factors. It has also been shown that HP1 recruitment to heterochromatin does not depend on Ago2 or Piwi and that loss of a single piRNA cluster leads to HP1 redistribution on the whole genome (79). Thus the relationship between heterochromatin formation and the piRNA complex may be multi-faceted.
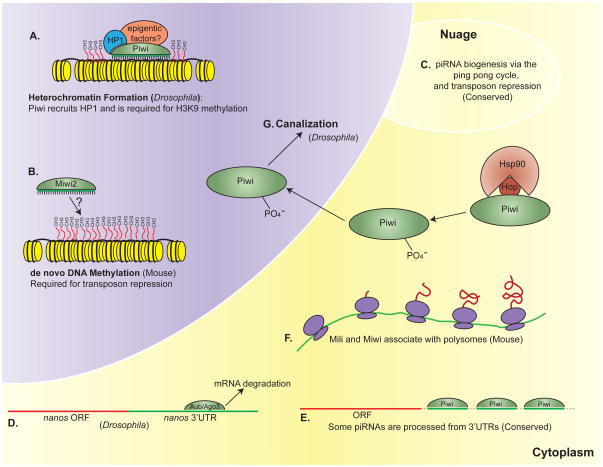
Figure 1. Molecular functions of Piwi proteins.
(A) In Drosophila, Piwi is a nuclear protein that binds Heterochromatin Protein 1a (HP1a) and is required for H3K9 (Lysine 9 of Histone 3) methylation and heterochromatin formation (10, 84). (B) In the mouse, Miwi2 protein is enriched in the nucleus and is required for de novo DNA methylation in male germ cells (4). (C) Piwi proteins in the nuage participate in a ping-pong mechanism that simultaneously produces new piRNAs and degrades active transposon mRNAs, see Figure 2 for details (9, 41). (D) The Drosophila Piwi proteins Aub and Ago3 are required for the deadenylation and degradation of maternal mRNAs during the maternal-to-zygotic transition (101). (E) In Drosophila, mouse, and Xenopus, 10–35% of piRNAs are processed from the 3′UTR of select mRNAs. This may represent a mechanism for regulating these mRNAs in cis. In addition, the resulting piRNAs may regulate gene expression in trans (100). (F) Mili and Miwi associate with polysomes in the mouse male germline to positively regulate gene expression (40, 117). (G) In Drosophila, Hsp90 and Hop form a complex with Piwi, which leads to Piwi phosphorylation and is required for Piwi-dependent canalization in the nucleus (36).
In the Mili and Miwi2 mutant mouse, loss of DNA methylation in TEs is observed, thus the epigenetic functions of Piwi proteins appear to be conserved in mammals (Figure 1B) (4, 58). Analysis of fetal germ cells revealed that Mili and Miwi2 play important roles in the establishment of de-novo DNA methylation to silence transposons (58). DNA methylation is catalyzed by Dnmt3L in the germline. However, piRNA production is not affected in the dnmt3l mutant, which indicates that the piRNA pathway functions upstream of DNA methylation (4). Future work is required to elucidate the molecular function of the piRNA pathway in transposon DNA methylation in the mouse male germline.
The Piwi-piRNA complex is required for genome integrity
Loss of Piwi proteins leads to germline-specific apoptosis, which may be triggered by DNA damage including, but not limited to, elevated transposition (13, 44, 52, 57). In the Miwi2 mutant mouse, the level of phosphorylated histone H2AX (γ-H2AX), which mark the sites of DNA double-stranded breaks that naturally occur during the leptotene stage of meiosis, is increased in zygotene-stage spermatocytes as compared to the wildtype cells (13). The failure to repair the double-stranded breaks and/or defective synapsis prevents germ cells from entering the pachytene stage of spermatogenesis and leads to apoptosis (13). However, despite increased levels of apoptosis, there is no significant difference in γ-H2AX staining between mili mutant and wildtype animals, suggesting an alternate pathway leading to apoptosis (57). In ziwi mutant zebrafish, increased levels of apoptosis are observed in the testes, but the prevalence of DNA double-stranded breaks have not been investigated (44). RIWI (rat Piwi) co-migrates with rRecQ1, an enzyme regulating DNA homologous recombination, which provides additional evidence that the Piwi/piRNA complex is involved in regulating genome integrity (45, 62).
In Drosophila, Aub and other piRNA pathway mutants lead to embryonic patterning defects and accumulation of γ-H2Av (equivalent to γ-H2Ax) foci in the germline (52). The embryonic patterning defects of piRNA pathway mutants, but not transposon de-repression, can be rescued by mutations in the Chk2 and ATR kinase components of the DNA damage-signaling pathway (52). The nature of the relationship between DNA damage and transposon mobility is currently unclear. One possibility is that transposon mobilization directly leads to DNA double-stranded breaks. However, it is also possible that double-stranded breaks induce transposon mobilization. In support of this, DNA damage can lead to transposon de-silencing in cell culture, and transposon mobilization can lead to double-stranded break repair (32, 78, 102). In addition, Aub is required for the production of telomere-specific piRNAs that may guide the telomere protection complex, which prevents chromosome fusion during mitosis and meiosis (48). Therefore, the piRNA pathway has functions in maintaining genome integrity that are separable from transposon repression.
Piwi proteins in regulating mRNA turnover
Several Piwi proteins are expressed predominantly in the cytoplasm, including Aub and Ago3 in Drosophila and Mili and Miwi in mice (9, 27, 57), suggesting their role in posttranscriptional regulation. For example, Aub and Ago3 associate with germ plasm and Mili and Miwi associate with the nuage and chromatoid body in mice round spermatids (9, 56, 117). Both germ plasm and the chromatoid body are electron-dense cytoplasmic perinuclear organelles containing both proteins and RNAs. These analogous structures, collectively called the nuage (cloud in French), contain many RNAs and RNA binding proteins (103). The nuage is common to animal germ cells, but its function remains enigmatic. It is thought to be a site of RNA storage and/or degradation. Accumulating evidence suggests that Piwi proteins are integral parts of the nuage where they function in post-transcriptional gene regulation.
Piwi proteins regulate the degradation of transposon mRNAs
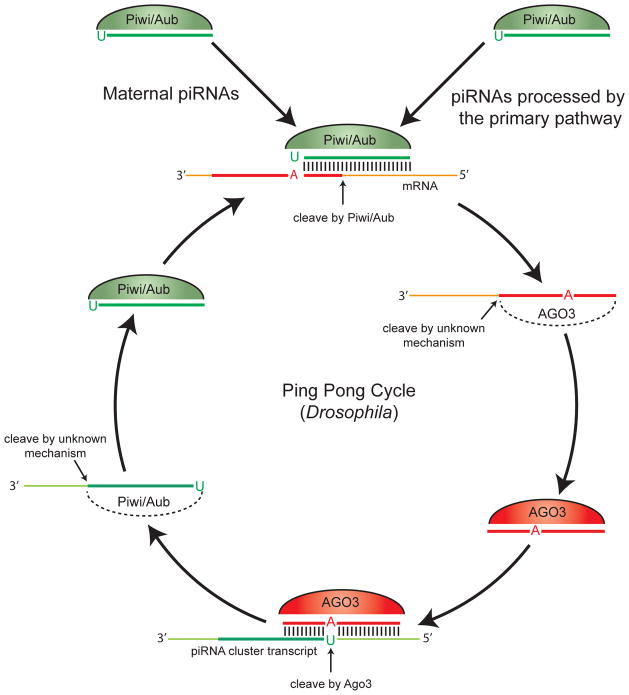
Piwi proteins are required to repress transposition in the Drosophila germline, because in piwi, aub, or ago3 mutant flies the expression of transposon RNAs increases significantly (54, 65, 118). Deep sequencing and characterization of subpopulations of piRNAs bound to each Drosophila Piwi protein led to the “ping-pong” hypothesis of piRNA biogenesis and transposon repression (Figure 2) (9, 41). This hypothesis is based on two key differences observed when comparing the piRNAs that bind Piwi and Aub versus those that bind Ago3. First, the majority of the piRNAs that bind Piwi and Aub are antisense to transposons whereas the majority of Ago3 bound piRNAs are sense. Second, Piwi/Aub piRNAs demonstrate a strong 5′uridine bias, while the majority of Ago3 piRNAs have an adenine at the 10th position. Piwi/Aub piRNAs and Ago3 piRNAs are often complimentary in the first 10 bases of their 5′ends; in particular 48% of sequenced Ago3 piRNAs had complimentary partners among the sequenced Aub piRNAs (9). These characteristics, collectively called the “ping pong signature,” support a model in which Piwi proteins generate the 5′ ends of new piRNAs from both piRNA primary transcripts and active mRNA transposons (Figure 2). In ago3 mutant flies, the number of Aub-bound piRNAs was significantly reduced and the antisense bias of these piRNAs is lost, thus providing experimental evidence for the ping-pong model (65).
Figure 2. The Ping Pong model of piRNA biogenesis and transposon repression in Drosophila (9, 41).
Piwi proteins generate the 5′ ends of new piRNAs that are derived from either piRNA cluster transcripts (primary piRNAs) or active mRNA transposons (secondary piRNAs). Piwi- and Aub-bound primary piRNAs are either maternally loaded or processed by the primary pathway. Next, Piwi and Aub, guided by their bound antisense piRNAs, bind to active transposon mRNAs and create the new 5′ ends of sense secondary piRNAs. Cleavage of the transposon mRNA occurs 10 nucleotides upstream of the adenine that is complimentary to the 5′uridine of the primary piRNA. Ago3 proteins, bound to secondary piRNAs, are then capable of producing the 5′ end of new primary piRNAs made from piRNA cluster transcripts. It is not known how the 3′ end of piRNAs are created in the ping pong mechanism.
The coupling of piRNA biogenesis to the degradation of transposon mRNAs may allow the piRNA pathway to react specifically to active transposon expression. Although the details of this model are best understood in Drosophila, it appears to be a conserved mechanism for controlling transposon expression in animals. In the mouse, evidence for the ping-pong mechanism is seen in pre-natal male germ cells when both Mili and Miwi2 are expressed. Mili-bound primary piRNAs (5′U bias) are predominantly sense to transposons whereas Miwi2-bound secondary piRNAs (10th position A bias) are mostly antisense (4). Therefore a “reverse ping-pong” is occurring in mice where primary piRNAs are made from transposon transcripts and secondary piRNAs are made from piRNA cluster transcripts. Deep sequencing of total putative piRNA populations in several animals, including zebrafish and planarians, has revealed ping-pong signatures (33, 44, 85). Post-transcriptional silencing of selfish genetic elements is likely conserved in the animal germline, but the mechanistic details have yet to be elucidated beyond Drosophila and mice. Given the differences observed between these two animals, it will be informative to uncover how the ping-pong pathway operates in other animals.
A commonality between mouse and Drosophila is the localization of the piRNA pathway proteins to the nuage, suggesting that this organelle is at least involved in piRNA biogenesis and transposon repression (Figure 1C). Protein localization data in mouse pre-natal male germ cells revealed that granules accumulating Mili and TDRD1 (“pi-bodies”) are distinct from granules accumulating Miwi2, TDRD9, and Maelstrom (“piP-bodies”) (6). piP-bodies are so named because of the coincident co-accumulation with known components of the processing (P)-body, a well-characterized site of mRNA translational repression and degradation (87). pi-bodies and piP-bodies are often found immediately adjacent to each other, but not overlapping, thus demonstrating compartmentalization of the piRNA pathway components (6). Mili, which binds primary piRNAs, is required for the formation of piP-bodies, as assessed by Miwi2 and Mael localization, which demonstrates the potential interplay between these two cytoplasmic structures (6, 13). Similar to mice, in the Drosophila ovary, several piRNA pathway components are dependent upon Aub and Ago3 expression for their localization to the germ plasm (65, 66). Taken together, these data suggest that the piRNA pathway may be one of the central functions of the germ cell nuage.
Piwi proteins regulate the degradation of non-transposon mRNAs
Piwi proteins have a prominent role in protecting the germline from selfish genetic elements. However, it is not clear if the myriad of phenotypes observed in various piwi mutant animals is due entirely to the de-repression of transposons. The piRNA pathway may also regulate the expression of non-transposon genes either transcriptionally (as discussed above) or post-transcriptionally. In support of this possibility, Rouget and coworkers demonstrate that Aub and Ago3 are required for the clearing of maternal mRNAs in preparation for the Drosophila maternal-to-zygotic transition (Figure 1D) (101). Aub and Ago3 are in a complex with the CCR4-NOT deadenylation complex and the localization of these proteins to small granules in the bulk cytoplasm is dependent upon the expression of piRNA pathway components. Several transposon-derived piRNAs are complimentary to sequences in the nanos 3′UTR and these putative binding sites are necessary for deadenylation and degradation of the mRNA. Injection of sequences antisense to these particular piRNAs results in the same head defects seen in piRNA pathway mutants, which is likely due to a failure to clear nanos mRNA, a posterior determinant, from the anterior (22, 64, 101). These data support a model in which Piwi proteins with their bound piRNAs recruit the deadenylation machinery to the nanos 3′UTR in the bulk cytoplasm, thus destabilizing the mRNA. This phenomenon is not specific to nanos, as several other mRNAs that are degraded at the maternal-to-zygotic transition have potential transposon-derived binding sites in their 3′UTR and depend on the piRNA pathway for their deadenylation (101). Therefore, piRNAs derived from transposon sequences are being used to regulate early development, thus demonstrating potential co-evolution between transposon sequences and the host genome (101).
It remains to be determined if piRNA pathway-mediated mRNA degradation of non-transposons is a prevalent paradigm for gene regulation in animals. Large numbers of piRNAs are not derived from transposon sequences, but rather map to diverse genomic regions, suggesting that Piwi proteins have functions in controlling gene expression that go beyond transposon control. For example, 10% of small RNAs in Drosophila OSS cell lines (ovary somatic sheet cells) are piRNAs derived from the 3′UTRs of select genes (Figure 1E) (100). Only the primary piRNA pathway operates in these cells, and accordingly these piRNAs have a 5′U bias (61, 100). Furthermore, the abundance of these piRNAs are not reduced in ago3 mutant flies, but are dramatically reduced in piwi mutant flies (100). In mouse 10-dpp testes, 35% of total small RNAs are derived from the 3′UTR of select genes. These are also likely produced by the primary pathway, as evidenced by their 5′U bias and dependence on mili expression (4, 100). There appears to be a selection mechanism in place to choose which 3′UTRs will be processed into piRNAs. For example, genes involved in developmental and regulatory processes are more highly represented than abundant house keeping genes (100). These piRNAs could be acting in cis to de-stabilize their mRNAs of origin, or in trans to regulate other genes either transcriptionally or post-transcriptionally (100). The significance of this discovery is not yet understood, but it does appear to be a conserved phenomenon with potential developmental importance.
Piwi proteins as positive translational regulators
Given that Mili and Miwi are associated with polysomes in the mouse male germline and that the association is correlated to the translational activity of the cells, Piwi proteins may also positively regulate translation (Figure 1F) (39, 117). In support of this notion, Mili interacts with eukaryotic initiation factor 3a (eIF3a) in an RNA-dependent manner. In mili mutant testes, the level of protein expression is decreased by 60% without a significant change in mRNA levels (117). This suggests that Mili acts as a general positive regulator in mouse testes. Miwi interacts generally with mRNAs in polysomes, which was shown both with an RNP-capture assay and with a cap-binding assay (40). Miwi mutant mice have decreased levels of specific mRNAs, which are targets of the spermatogenic transcriptional activator CREM (cAMP-responsive element upmodulator) (27). This suggests that Miwi binding to these messages may be required to stabilize and/or protect these mRNAs from degradation for translation. In this way, Miwi may also act as a positives translational regulator in the mouse male germline. It is possible that the putative role of Piwi proteins as positive translational regulators is conserved beyond the mouse germline. In Drosophila, aubergine is required to enhance Oskar translation (126). The Xenopus Piwi protein, Xiwi, interacts with poly-A-binding protein (ePAB) and small ribosomal proteins, but no role for Xiwi in translational regulation has yet been identified (60). These data suggest that further investigation of Piwi function in translational activation is warranted and may uncover a conserved and critical function of Piwi proteins.
The piRNA pathway and developmental robustness
Several lines of evidence suggest that Piwi proteins act as epigenetic regulators, but little is understood about the function of this epigenetic regulation during development. Recent studies in our lab show that in Drosophila Piwi and Aub are required for canalization, which is an organism’s ability to produce the same phenotype despite inherent genetic variations and environmental influences (36). Piwi forms a multiprotein complex with Hsp90 and Hop, which directs phosphorylation of Piwi in the cytoplasm. The phorphorylated Piwi then enters the nucleus to suppress the expression of hidden genetic variation and transposon activity. (Figure 1G) (36, 112). Hsp90 and Hop may regulate the post-translational modifications of Piwi proteins to function in epigenetic silencing and mediate canalization (36). This ensures developmental robustness of individual organisms for natural selection, but also may allow an increase in genetic and epigenetic variability during times of stress to promote phenotypic diversity for selection.
Evolutionary Perspective: An ancient function for Piwi proteins in germline and stem cells
Fungi, plants, and green algae contain only Argonaute subfamily proteins whereas animals have both Argonaute and Piwi subfamily proteins (14). Some unicellular organisms such as Amoebozoa and the ciliated protist Tetrahymena thermophila have Piwi subfamily, but not Argonaute subfamily proteins (14). This suggests that Piwi proteins are not an invention of multicellular animals, but rather have a more ancient origin. Studies in a wide variety of both unicellular organisms and multicellular animals at key phylogenetic positions will allow us to ascertain the conserved and fundamental functions of Piwi proteins.
The function Piwi proteins during Tetrahymena sexual reproduction
The genome of Tetrahymena thermophila contains twelve putative Piwi proteins, twi1-twi12. Eight of these are known to be expressed and to bind to diverse populations of small RNAs with distinct sizes, genomic origins, genetic requirements for accumulation, and timing of expression during the life cycle (21). Tetrahymena undergoes both sexual (conjugation) and asexual (vegetative growth) reproduction (15). During vegetative growth two nuclei are maintained: a silent diploid “germline” micronucleus, which contains the complete genome, and an active polyploidy “somatic” macronucleus, which has undergone region-specific DNA elimination. During conjugation, the germline nuclei undergo meiosis, gamete exchange, and fusion. The resulting zygotic nucleus undergoes mitosis and somatic differentiation. This process requires TWI1, which binds to a class of small RNAs called scan RNAs (76). It is proposed that scan RNAs are made in the germline nucleus and then bind to TWI1 protein in the cytoplasm. The TWI1/scan RNA complex subsequently moves to the new somatic nucleus and marks DNA for elimination with an H3K9 methylation (74). As discussed above, Drosophila Piwi is required for H3K9 methylation and heterochromatin formation, thus suggesting a conserved function for Piwi family proteins in directing histone modifications (10). In support of this, scan RNAs are similar to piRNAs in size (27–30 nucleotides) and they have a 2′-O-methylation (59, 76). On the other hand, scan RNA production requires the Dicer ortholog, dcl1, while animal piRNA production is Dicer-independent (44, 77). Regardless, it is clear that Tetrahymena requires Piwi proteins to complete its sexual cycle, which offers an interesting comparison with animal Piwi proteins.
Piwi genes as stem cell genes in non-bilatarians
Piwi genes have been identified in representative species of several bilaterian sister groups, which suggests that Piwi proteins were present in the last common ancestor of all bilatarians (Figure 3) (2, 28, 34, 108). Non-bilataria Phylums include Porifera (sponges), Ctenophora (comb jellies), and Cnidaria (jelly fish, sea anemones, and corals). The evolutionary relationships between these species is controversial, but the cnidarians are the well-accepted sister group to the bilaterians (30, 89, 90). In non-bilatarian metazoans, piwi expression is not limited to the germline, but instead functions more broadly in stem cells. For example, in the sponge Ephydatia fluviatilis, two piwi orthologs are selectively expressed in the archeocytes, adult stem cells that give rise to both differentiated somatic and germ cells (34). In the cnidarian, Podocoryne carnea, a piwi homolog is expressed in the germline, as well as in transdifferentiating tissues (108). In the cnidarian, Clytia hemisphaerica, a piwi homolog is expressed in stem cells of the medusa tentacle bulb that give rise to nematocytes (cnidarian stinging cells) (28). In the ctenophore Pleurobrachia pileus, two piwi homologs are expressed in the germline as well as in several somatic domains (2). These somatic domains are sites of active cell proliferation and are likely stem cells that give rise to various differentiated adult tissues (2).
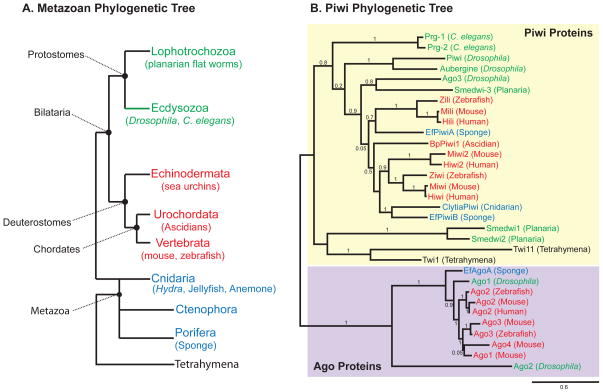
Figure 3. Piwi proteins are conserved in metazoans.
(A) A metazoan phylogenetic tree. Bilatarians are classified as either protostomes (green) or deuterostomes (red) based on differences in embryo development. Non-bilatarian phyla (blue) include cnidarians, ctenophores, and sponges. Tetrahymena is a ciliated protist that also expresses Piwi proteins. (B) Phylogenetic analysis of Argonaute proteins demonstrates a clear distinction between the Piwi (yellow) and Argonuate (purple) subfamilies. Phylogram generated using Phylogeny.fr (29). Numbers on the tree demonstrate branch supports. NCBI accession numbers from top to bottom are as follows: Prg-1 (NM_059720), Prg-2 (NM_068593), Piwi (NM_057527), Aubergine (NM_001103674), Ago3 (NM_001043162), Smedwi-2 (EU586258), Zili (NM001080199), Mili (NM_021308), Hili (NM_001135721), EfPiwiA (AB533505), BpPiwi1 (AB455103), Miwi2 (NM_177905), Hiwi2 (NM_152431), Ziwi (NM_18338), Miwi (NM_021311), Hiwi (NM_004764.4), ClytiaPiwi (EU199802), EfPiwiB (AB533506.1), Smedwi1 (DQ186985), Smedwi2 (DQ186986), Twi11 (EU183125), Twi1 (AB084111), EfAgoA (AB533507), Drosophila Ago1 (NM_166020), Zebrafish Ago2 (XM_694134), Mouse Ago2 (AY135688), Human Ago2 (NM_001164623), Mouse Ago3 (AY135689), Zebrafish Ago3 (XM_690923), Mouse Ago4 (AY135690), Mouse Ago1 (AY135687), Drosophila Ago2 (NM_140518).
To determine if bilatarian-type piRNAs exist in animals such as the cnidarians or sponges, total small RNA populations were sequenced from the sponge Amphimedon queenslandica and the starlet sea anemone Nematostella vectensis (38). A large fraction of the small RNAs found in both animals were approximately 27 nucleotides long and were resistant to periodate oxidation implying the existence of a 2′-O –methylation. These small RNAs have a 5′ uridine bias and a fraction of them come from clustered genomic loci (45% in Nematostella and 10% in Amphimedon) (38). Therefore, these are likely primary piRNAs, although true classification of piRNAs requires a demonstrated association with Piwi proteins. In addition, they found that candidate piRNAs that were sense to the mRNAs (~35% corresponding to transposons) had a bias for a 10th position adenine, thus providing evidence for ping-pong piRNA synthesis in these non-bilatarian animals (38).
As described in this review, the majority of our understanding of Piwi protein function comes from Drosophila and mouse. These studies demonstrate diverse roles for Piwi in controlling gene expression at several levels, both transcriptional and post-transcriptional. However, it remains unclear how these molecular functions contribute to the maintenance of germline stem cells. Non-bilatarian phyla offer an intriguing position on the phylogenetic tree for interrogating Piwi function. Given the common suite of genes (e.g. vasa, nanos, and piwi), the molecular functions of Piwi proteins in the stem cells of non-bilatarian metazoans will likely be conserved in, for example, the germline of Drosophila and mice (47). Furthermore, understanding the function of Piwi proteins in non-bilatarian animals may uncover the most conserved functions of these proteins in stem cells, thus shedding light on the unifying mechanisms common to all animals. Thus far, studies of Piwi proteins in early branching metazoans have been restricted to expression analyses. However, in two cnidarian model systems, Hydra and Nematostella vectensis, modern molecular tools are now being applied to study gene function, including gene knockdown by RNAi or morpholinos, the generation of transgenic animals, and genome sequencing. Thus, researchers are now in a position to interrogate Piwi function in these animals (16, 19, 92, 96, 127).
The role of piwi genes in bilatarian adult stem cells
Bilatarians are split into two major groups based on the development of their embryos, the deuterostomes and protostomes (Figure 3). The deuterostomes include the vertebrates as well as some invertebrate groups (e.g. echinoderms and tunicates). The tunicates are a subphylum of the chordates, and are the sister group to vertebrates (26, 30). In one tunicate group, the Botrylloides colonial ascidians, a piwi homolog is expressed in stem cells capable of giving rise to all three germ layers during whole body regeneration (11, 99). In Botrylloides leachi, RNAi knockdown demonstrates the requirement of piwi for regeneration in this animal (99). Thus, piwi may have an ancient association with stemness in the deuterostome lineage. The protostome branch of the Bilataria is split into two groups: the ecdysozoans, molting animals, and the lophotrochozoans, which are loosely grouped together based in part on embryological and larval similarities (Figure 3) (30). Two very successful model systems, Drosophila and C. elegans, are ecdysozoans, and therefore a large amount of functional data on Piwi proteins comes from this group. Functional data from the lophotrochozoan clade is largely missing, with one exception being the flatworms where RNAi is routinely used to knockdown gene function (80). Flatworms contain totipotent adult stem cells, the neoblasts, which give rise to all somatic and germ cell types, thus giving flatworms remarkable regeneration capabilities (7, 81). In the planarian flatworm Schmidtea mediterranea two piwi homologs, Smedwi-2 and Smedwi-3, are expressed in the neoblasts and are required for neoblast function in regeneration (85, 95). Deep sequencing studies in planaria revealed the existence of both primary and secondary putative piRNAs (33, 85). In the marine flatworm Macrostomum lignano, a piwi homolog is required for the maintenance of neoblasts, reminiscent of its function in maintaining GSCs in Drosophila (22, 25). Taking the evidence from both traditional and non-traditional model organisms together suggests that Piwi proteins have an ancient function in regulating stem cells (47).
The impact of Piwi proteins on human health
The broad phylogenetic survey of the expression patterns of piwi genes described above supports a conserved function for Piwi proteins in the stem cells of both somatic and germline tissues. A human piwi ortholog, hiwi, is expressed in hematopoietic stem and progenitor cells, but not in the differentiated products of these cells, thus suggesting a role for Piwi proteins in at least one type of human somatic stem cells (109). In addition to the potential function for Piwi proteins in normal somatic stem cells, several studies demonstrate a correlation of ectopic hiwi or hili expression with the occurrence of several cancer types, including, but not limited to, testicular seminomas, breast cancer, soft tissue sarcomas, gastric cancer, and ovarian cancer (63, 70, 73, 94, 114).
Stem cells and cancer cells share several characteristics including the ability to self-renew and relative resistance to drugs and radiation. It is hypothesized that in some cases a de-regulation of normal stem cells will transform them into cancer stem cells (CSCs), and that the creation of CSCs is required for transformation from non-malignancy to malignancy (98). Thus, the identification of stem cell-associated genes in cancerous tissue may reflect the existence of CSCs. Several studies demonstrate that Piwi proteins are required for the continued proliferation of cancer cells, which is similar to their requirement for GSC proliferation in Drosophila (18, 23, 63, 73). This supports the hypothesis that cancerous cells appropriate the stem cell machinery to promote their continued survival. In Drosophila, brain tumors caused by temperature sensitive mutations in the lethal (3) malignant brain tumor [l(3)mbt] gene ectopically express a significant number of genes required for germline formation and maintenance, including Piwi and Aubergine (46). The average brain tumor size is significantly reduced when piwi or aub mutations are introduced. Therefore, future studies should focus on the functions of stem cell genes such as the Piwi family in CSCs, as interfering with these functions may be a strategy to develop therapies.
Conclusion
Current studies of the Piwi/piRNA pathway have revealed its diverse biological and molecular functions, including germline specification, gametogenesis, stem cell maintenance, epigenetic programming, transposon silencing, genome protection, and posttranscriptional regulation of mRNAs. Among these discoveries, perhaps the most extensively reported one is its role in repressing transposable elements in the germline both at the transcriptional and post-transcriptional levels (106). Despite these reports, this process remains mysterious in many ways. For example, the production of primary piRNAs, such as the transcriptional control and processing of the precursors, is not understood. In addition, the details of the quality control mechanism used to select mRNAs (often transposon mRNAs) for the production of secondary piRNAs are not understood. It is also not clear how the desilencing of transposons might be connected to the various developmental phenotypes observed for piwi mutants, such as GSC loss and embryonic patterning defects in Drosophila, GSC loss and spermatogenic arrest in mice, germ cell death in zebrafish, and inhibition of stem cell function in planarians (13, 23, 27, 42, 44, 57, 65). It is possible that transposon activation triggers regulatory pathways, which in turn leads to these aberrant phenotypes.
There is evidence that the Piwi/piRNA complex functions in epigenetic control, but many aspects of this mechanism remain elusive. For example, only HP1 has been found to be involved in heterochromatin formation with Piwi proteins. What is the relationship between histone methyltransferase, DNA methyltransferase and Piwi in epigenetic regulation? If piRNAs target Piwi proteins to the chromosomes, do they bind DNA sequences or nascent transcripts? At the level of epigenetic regulation, how do piRNAs distinguish transposon sequences and protein coding genes? The answers to these questions are important because the Piwi/piRNA complex may act to select specific chromosomal regions to be targeted by more general epigenetic factors.
There is also evidence that the Piwi/piRNA pathway regulates non-transposon genes post-transcriptionally in Drosophila, such as the targeting of maternal genes for deadenylation at the maternal-to-zygotic transition (101). Given that a significant subpopulation of piRNAs do not map to transposons, future research is likely to uncover new instances of Piwi/piRNA regulation of non-transposon genes. To harness the therapeutic power of stem cells, we need to understand precisely the underlying molecular circuitry governing their cell fate decisions. To accomplish this goal, a number of model organisms have been instrumental in the identification of genes and pathways involved in stem cell regulatory control. The Piwi/piRNA pathway is required for maintaining stem cells in the animal germline and perhaps in a wider variety of stem cells, including in humans (23, 109). Understanding the full regulatory capabilities of the Piwi/piRNA pathway will be essential for understanding how it regulates stem cells and other developmental functions.
SUMMARY POINTS.
Piwi proteins bind to piRNAs and function in both germ cells and stem cells throughout animal phylogeny.
piRNAs are produced via a dicer-independent mechanism in two steps: Primary piRNAs are made by an unknown mechanism from long single-stranded precursors. Secondary piRNAs are then made by the ping-pong mechanism in which Piwi proteins cleave piRNA cluster transcripts or active mRNAs into piRNAs.
Piwi proteins maintain fertility in Drosophila, C. elegans, and mouse, at least in part through maintenance of the germline stem cells.
Piwi proteins repress transposition in the animal germline, presumably by both epigenetic suppression and degradation of transposon mRNA (e.g., via ping-pong mechanism).
Piwi proteins bind to Tudor-domain containing proteins in the nuage of germ cells. Tudor-domain containing proteins may function to ensure quality control of the mRNAs selected for piRNA biogenesis by Piwi proteins.
Some Piwi proteins are in the nucleus and function in epigenetic mechanisms, including histone modification (Drosophila) and DNA methylation (mouse).
Some Piwi proteins are in the cytoplasm, where they are often localized to the nuage and function in post-transcriptional gene regulation.
Piwi function is not limited to animal germlines, but instead is required more broadly in stem cells in diverse organisms.
FUTURE ISSUES.
Many questions remain open regarding Piwi/piRNA control of transposable elements. For example, how is the transcription of piRNA precursors regulated? What is the mechanism of primary piRNA production? And, how are specific mRNAs selected for secondary piRNA biogenesis?
The developmental phenotype of piwi mutants are varied and complex. What molecular mechanism mediated by Piwi is related to a specific phenotype? e.g., is a phenotype due to the miss-regulation of transposons or other genes, or both?
What is the relationship between Piwi proteins and other epigenetic regulators? Do Piwi/piRNA complexes bind to DNA or to nascent RNA transcripts? How do Piwi/piRNA complexes recognize specific genomic sequences, for example, distinguish transposons from non-transposons in the genome?
What is the function of Piwi/piRNA complexes that do not target transposons for repression? How prevalent is Piwi-directed regulation of non-transposon genes, and what other processes do Piwi/piRNA complexes regulate?
Acknowledgments
Current work in the Lin lab is supported by the NIH (DP1OD006852, R01HD37760S1, and R01HD42042), the G. Harold and Leila Mathers Foundation, theConnecticut Stem Cell Research Funds (08-SCD-Yale-004), and the Ellison Medical Foundation to H.L. C.J. is a NRSA Postdoctoral Fellow (NIH F32GM090372).
ACRONYMS
- miRNAs
microRNAs
- siRNAs
small-interfering RNAs
- piRNAs
piwi-interacting RNAs
- GCS
Germline stem cell
- PGC
Primordial germ cell
- CSC
Cancer stem cell
- TE
Transposable elements
- PEV
Position effect variegation
- P-body
Processing body
- MZT
Maternal to zygotic transition
LITERATURE CITED
- 1.Agata K, Nakajima E, Funayama N, Shibata N, Saito Y, Umesono Y. Two different evolutionary origins of stem cell systems and their molecular basis. Semin Cell Dev Biol. 2006;17:503–9. doi: 10.1016/j.semcdb.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Alie A, Leclere L, Jager M, Dayraud C, Chang P, et al. Somatic stem cells express Piwi and Vasa genes in an adult ctenophore: Ancient association of “germline genes” with stemness. Dev Biol. 2011;350:183–97. doi: 10.1016/j.ydbio.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 3.Aravin A, Gaidatzis D, Pfeffer S, Lagos-Quintana M, Landgraf P, et al. A novel class of small RNAs bind to MILI protein in mouse testes. Nature. 2006;442:203–7. doi: 10.1038/nature04916. [DOI] [PubMed] [Google Scholar]
- 4.Aravin AA, Sachidanandam R, Bourc’his D, Schaefer C, Pezic D, et al. A piRNA pathway primed by individual transposons is linked to de novo DNA methylation in mice. Mol Cell. 2008;31:785–99. doi: 10.1016/j.molcel.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aravin AA, Sachidanandam R, Girard A, Fejes-Toth K, Hannon GJ. Developmentally regulated piRNA clusters implicate MILI in transposon control. Science. 2007;316:744–7. doi: 10.1126/science.1142612. [DOI] [PubMed] [Google Scholar]
- 6.Aravin AA, van der Heijden GW, Castaneda J, Vagin VV, Hannon GJ, Bortvin A. Cytoplasmic compartmentalization of the fetal piRNA pathway in mice. PLoS Genet. 2009;5:e1000764. doi: 10.1371/journal.pgen.1000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baguna J, Salo E, Auladell C. Regeneration and pattern formation in planarians III. Evidence that neoblasts are totipotent stem cells and the source of blastema cells. Development. 1989;107:77–86. [Google Scholar]
- 8.Boswell RE, Mahowald AP. tudor, a gene required for assembly of the germ plasm in Drosophila melanogaster. Cell. 1985;43:97–104. doi: 10.1016/0092-8674(85)90015-7. [DOI] [PubMed] [Google Scholar]
- 9.Brennecke J, Aravin AA, Stark A, Dus M, Kellis M, et al. Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell. 2007;128:1089–103. doi: 10.1016/j.cell.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 10.Brower-Toland B, Findley SD, Jiang L, Liu L, Yin H, et al. Drosophila PIWI associates with chromatin and interacts directly with HP1a. Genes Dev. 2007;21:2300–11. doi: 10.1101/gad.1564307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown FD, Keeling EL, Le AD, Swalla BJ. Whole body regeneration in a colonial ascidian, Botrylloides violaceus. J Exp Zool B Mol Dev Evol. 2009;312B:885–900. doi: 10.1002/jez.b.21303. [DOI] [PubMed] [Google Scholar]
- 12.Buhler M, Verdel A, Moazed D. Tethering RITS to a nascent transcript initiates RNAi- and heterochromatin-dependent gene silencing. Cell. 2006;125:873–86. doi: 10.1016/j.cell.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 13.Carmell MA, Girard A, van de Kant HJ, Bourc’his D, Bestor TH, et al. MIWI2 is essential for spermatogenesis and repression of transposons in the mouse male germline. Dev Cell. 2007;12:503–14. doi: 10.1016/j.devcel.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Cerutti H, Casas-Mollano JA. On the origin and functions of RNA-mediated silencing: from protists to man. Curr Genet. 2006;50:81–99. doi: 10.1007/s00294-006-0078-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chalker DL. Dynamic nuclear reorganization during genome remodeling of Tetrahymena. Biochim Biophys Acta. 2008;1783:2130–6. doi: 10.1016/j.bbamcr.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chapman JA, Kirkness EF, Simakov O, Hampson SE, Mitros T, et al. The dynamic genome of Hydra. Nature. 2010;464:592–6. doi: 10.1038/nature08830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen C, Jin J, James DA, Adams-Cioaba MA, Park JG, et al. Mouse Piwi interactome identifies binding mechanism of Tdrkh Tudor domain to arginine methylated Miwi. Proc Natl Acad Sci U S A. 2009;106:20336–41. doi: 10.1073/pnas.0911640106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen L, Shen R, Ye Y, Pu XA, Liu X, et al. Precancerous stem cells have the potential for both benign and malignant differentiation. PLoS One. 2007;2:e293. doi: 10.1371/journal.pone.0000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chera S, de Rosa R, Miljkovic-Licina M, Dobretz K, Ghila L, et al. Silencing of the hydra serine protease inhibitor Kazal1 gene mimics the human SPINK1 pancreatic phenotype. J Cell Sci. 2006;119:846–57. doi: 10.1242/jcs.02807. [DOI] [PubMed] [Google Scholar]
- 20.Chuma S, Hiyoshi M, Yamamoto A, Hosokawa M, Takamune K, Nakatsuji N. Mouse Tudor Repeat-1 (MTR-1) is a novel component of chromatoid bodies/nuages in male germ cells and forms a complex with snRNPs. Mech Dev. 2003;120:979–90. doi: 10.1016/s0925-4773(03)00181-3. [DOI] [PubMed] [Google Scholar]
- 21.Couvillion MT, Lee SR, Hogstad B, Malone CD, Tonkin LA, et al. Sequence, biogenesis, and function of diverse small RNA classes bound to the Piwi family proteins of Tetrahymena thermophila. Genes Dev. 2009;23:2016–32. doi: 10.1101/gad.1821209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cox DN, Chao A, Baker J, Chang L, Qiao D, Lin H. A novel class of evolutionarily conserved genes defined by piwi are essential for stem cell self-renewal. Genes Dev. 1998;12:3715–27. doi: 10.1101/gad.12.23.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cox DN, Chao A, Lin H. piwi encodes a nucleoplasmic factor whose activity modulates the number and division rate of germline stem cells. Development. 2000;127:503–14. doi: 10.1242/dev.127.3.503. [DOI] [PubMed] [Google Scholar]
- 24.Creed TM, Loganathan SN, Varonin D, Jackson CA, Arkov AL. Novel role of specific Tudor domains in Tudor-Aubergine protein complex assembly and distribution during Drosophila oogenesis. Biochem Biophys Res Commun. 402:384–9. doi: 10.1016/j.bbrc.2010.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Mulder K, Pfister D, Kuales G, Egger B, Salvenmoser W, et al. Stem cells are differentially regulated during development, regeneration and homeostasis in flatworms. Dev Biol. 2009;334:198–212. doi: 10.1016/j.ydbio.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 26.Delsuc F, Brinkmann H, Chourrout D, Philippe H. Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature. 2006;439:965–8. doi: 10.1038/nature04336. [DOI] [PubMed] [Google Scholar]
- 27.Deng W, Lin H. miwi, a murine homolog of piwi, encodes a cytoplasmic protein essential for spermatogenesis. Dev Cell. 2002;2:819–30. doi: 10.1016/s1534-5807(02)00165-x. [DOI] [PubMed] [Google Scholar]
- 28.Denker E, Manuel M, Leclere L, Le Guyader H, Rabet N. Ordered progression of nematogenesis from stem cells through differentiation stages in the tentacle bulb of Clytia hemisphaerica (Hydrozoa, Cnidaria) Dev Biol. 2008;315:99–113. doi: 10.1016/j.ydbio.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 29.Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, et al. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008;36:W465–9. doi: 10.1093/nar/gkn180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dunn CW, Hejnol A, Matus DQ, Pang K, Browne WE, et al. Broad phylogenomic sampling improves resolution of the animal tree of life. Nature. 2008;452:745–9. doi: 10.1038/nature06614. [DOI] [PubMed] [Google Scholar]
- 31.Farazi TA, Juranek SA, Tuschl T. The growing catalog of small RNAs and their association with distinct Argonaute/Piwi family members. Development. 2008;135:1201–14. doi: 10.1242/dev.005629. [DOI] [PubMed] [Google Scholar]
- 32.Farkash EA, Kao GD, Horman SR, Prak ET. Gamma radiation increases endonuclease-dependent L1 retrotransposition in a cultured cell assay. Nucleic Acids Res. 2006;34:1196–204. doi: 10.1093/nar/gkj522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friedlander MR, Adamidi C, Han T, Lebedeva S, Isenbarger TA, et al. High-resolution profiling and discovery of planarian small RNAs. Proc Natl Acad Sci U S A. 2009;106:11546–51. doi: 10.1073/pnas.0905222106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Funayama N, Nakatsukasa M, Mohri K, Masuda Y, Agata K. Piwi expression in archeocytes and choanocytes in demosponges: insights into the stem cell system in demosponges. Evol Dev. 2010;12:275–87. doi: 10.1111/j.1525-142X.2010.00413.x. [DOI] [PubMed] [Google Scholar]
- 35.Gangaraju VK, Lin H. MicroRNAs: key regulators of stem cells. Nat Rev Mol Cell Biol. 2009;10:116–25. doi: 10.1038/nrm2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gangaraju VK, Yin H, Weiner MM, Wang J, Huang XA, Lin H. Drosophila Piwi functions in Hsp90-mediated suppression of phenotypic variation. Nat Genet. 2011;43:153–8. doi: 10.1038/ng.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grewal SI. RNAi-dependent formation of heterochromatin and its diverse functions. Curr Opin Genet Dev. 2010;20:134–41. doi: 10.1016/j.gde.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grimson A, Srivastava M, Fahey B, Woodcroft BJ, Chiang HR, et al. Early origins and evolution of microRNAs and Piwi-interacting RNAs in animals. Nature. 2008;455:1193–7. doi: 10.1038/nature07415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grivna ST, Beyret E, Wang Z, Lin H. A novel class of small RNAs in mouse spermatogenic cells. Genes Dev. 2006;20:1709–14. doi: 10.1101/gad.1434406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grivna ST, Pyhtila B, Lin H. MIWI associates with translational machinery and PIWI-interacting RNAs (piRNAs) in regulating spermatogenesis. Proc Natl Acad Sci U S A. 2006;103:13415–20. doi: 10.1073/pnas.0605506103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gunawardane LS, Saito K, Nishida KM, Miyoshi K, Kawamura Y, et al. A slicer-mediated mechanism for repeat-associated siRNA 5’ end formation in Drosophila. Science. 2007;315:1587–90. doi: 10.1126/science.1140494. [DOI] [PubMed] [Google Scholar]
- 42.Harris AN, Macdonald PM. Aubergine encodes a Drosophila polar granule component required for pole cell formation and related to eIF2C. Development. 2001;128:2823–32. doi: 10.1242/dev.128.14.2823. [DOI] [PubMed] [Google Scholar]
- 43.Houwing S, Berezikov E, Ketting RF. Zili is required for germ cell differentiation and meiosis in zebrafish. Embo J. 2008;27:2702–11. doi: 10.1038/emboj.2008.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Houwing S, Kamminga LM, Berezikov E, Cronembold D, Girard A, et al. A role for Piwi and piRNAs in germ cell maintenance and transposon silencing in Zebrafish. Cell. 2007;129:69–82. doi: 10.1016/j.cell.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 45.Hunter N. The RecQ DNA helicases: Jacks-of-all-trades or master-tradesmen? Cell Res. 2008;18:328–30. doi: 10.1038/cr.2008.33. [DOI] [PubMed] [Google Scholar]
- 46.Janic A, Mendizabal L, Llamazares S, Rossell D, Gonzalez C. Ectopic expression of germline genes drives malignant brain tumor growth in Drosophila. Science. 2010;330:1824–7. doi: 10.1126/science.1195481. [DOI] [PubMed] [Google Scholar]
- 47.Juliano CE, Swartz SZ, Wessel GM. A conserved germline multipotency program. Development. 2010;137:4113–26. doi: 10.1242/dev.047969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khurana JS, Xu J, Weng Z, Theurkauf WE. Distinct functions for the Drosophila piRNA pathway in genome maintenance and telomere protection. PLoS Genet. 2010;6:e1001246. doi: 10.1371/journal.pgen.1001246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim VN, Han J, Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol. 2009;10:126–39. doi: 10.1038/nrm2632. [DOI] [PubMed] [Google Scholar]
- 50.Kirino Y, Kim N, de Planell-Saguer M, Khandros E, Chiorean S, et al. Arginine methylation of Piwi proteins catalysed by dPRMT5 is required for Ago3 and Aub stability. Nat Cell Biol. 2009;11:652–8. doi: 10.1038/ncb1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kirino Y, Vourekas A, Sayed N, de Lima Alves F, Thomson T, et al. Arginine methylation of Aubergine mediates Tudor binding and germ plasm localization. Rna. 2010;16:70–8. doi: 10.1261/rna.1869710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klattenhoff C, Bratu DP, McGinnis-Schultz N, Koppetsch BS, Cook HA, Theurkauf WE. Drosophila rasiRNA pathway mutations disrupt embryonic axis specification through activation of an ATR/Chk2 DNA damage response. Dev Cell. 2007;12:45–55. doi: 10.1016/j.devcel.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 53.Klattenhoff C, Xi H, Li C, Lee S, Xu J, et al. The Drosophila HP1 homolog Rhino is required for transposon silencing and piRNA production by dual-strand clusters. Cell. 2009;138:1137–49. doi: 10.1016/j.cell.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klenov MS, Lavrov SA, Stolyarenko AD, Ryazansky SS, Aravin AA, et al. Repeat-associated siRNAs cause chromatin silencing of retrotransposons in the Drosophila melanogaster germline. Nucleic Acids Res. 2007;35:5430–8. doi: 10.1093/nar/gkm576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kojima K, Kuramochi-Miyagawa S, Chuma S, Tanaka T, Nakatsuji N, et al. Associations between PIWI proteins and TDRD1/MTR-1 are critical for integrated subcellular localization in murine male germ cells. Genes Cells. 2009;14:1155–65. doi: 10.1111/j.1365-2443.2009.01342.x. [DOI] [PubMed] [Google Scholar]
- 56.Kotaja N, Bhattacharyya SN, Jaskiewicz L, Kimmins S, Parvinen M, et al. The chromatoid body of male germ cells: similarity with processing bodies and presence of Dicer and microRNA pathway components. Proc Natl Acad Sci U S A. 2006;103:2647–52. doi: 10.1073/pnas.0509333103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuramochi-Miyagawa S, Kimura T, Ijiri TW, Isobe T, Asada N, et al. Mili, a mammalian member of piwi family gene, is essential for spermatogenesis. Development. 2004;131:839–49. doi: 10.1242/dev.00973. [DOI] [PubMed] [Google Scholar]
- 58.Kuramochi-Miyagawa S, Watanabe T, Gotoh K, Totoki Y, Toyoda A, et al. DNA methylation of retrotransposon genes is regulated by Piwi family members MILI and MIWI2 in murine fetal testes. Genes Dev. 2008;22:908–17. doi: 10.1101/gad.1640708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kurth HM, Mochizuki K. 2′-O-methylation stabilizes Piwi-associated small RNAs and ensures DNA elimination in Tetrahymena. RNA. 2009;15:675–85. doi: 10.1261/rna.1455509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lau NC, Ohsumi T, Borowsky M, Kingston RE, Blower MD. Systematic and single cell analysis of Xenopus Piwi-interacting RNAs and Xiwi. Embo J. 2009;28:2945–58. doi: 10.1038/emboj.2009.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lau NC, Robine N, Martin R, Chung WJ, Niki Y, et al. Abundant primary piRNAs, endo-siRNAs, and microRNAs in a Drosophila ovary cell line. Genome Res. 2009;19:1776–85. doi: 10.1101/gr.094896.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lau NC, Seto AG, Kim J, Kuramochi-Miyagawa S, Nakano T, et al. Characterization of the piRNA complex from rat testes. Science. 2006;313:363–7. doi: 10.1126/science.1130164. [DOI] [PubMed] [Google Scholar]
- 63.Lee JH, Schutte D, Wulf G, Fuzesi L, Radzun HJ, et al. Stem-cell protein Piwil2 is widely expressed in tumors and inhibits apoptosis through activation of Stat3/Bcl-XL pathway. Hum Mol Genet. 2006;15:201–11. doi: 10.1093/hmg/ddi430. [DOI] [PubMed] [Google Scholar]
- 64.Lehmann R, Nusslein-Volhard C. The maternal gene nanos has a central role in posterior pattern formation of the Drosophila embryo. Development. 1991;112:679–91. doi: 10.1242/dev.112.3.679. [DOI] [PubMed] [Google Scholar]
- 65.Li C, Vagin VV, Lee S, Xu J, Ma S, et al. Collapse of germline piRNAs in the absence of Argonaute3 reveals somatic piRNAs in flies. Cell. 2009;137:509–21. doi: 10.1016/j.cell.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lim AK, Kai T. Unique germ-line organelle, nuage, functions to repress selfish genetic elements in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2007;104:6714–9. doi: 10.1073/pnas.0701920104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lin H. Cell biology of stem cells: an enigma of asymmetry and self-renewal. J Cell Biol. 2008;180:257–60. doi: 10.1083/jcb.200712159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lin H, Spradling AC. A novel group of pumilio mutations affects the asymmetric division of germline stem cells in the Drosophila ovary. Development. 1997;124:2463–76. doi: 10.1242/dev.124.12.2463. [DOI] [PubMed] [Google Scholar]
- 69.Liu H, Wang JY, Huang Y, Li Z, Gong W, et al. Structural basis for methylarginine-dependent recognition of Aubergine by Tudor. Genes Dev. 2010;24:1876–81. doi: 10.1101/gad.1956010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu JJ, Shen R, Chen L, Ye Y, He G, et al. Piwil2 is expressed in various stages of breast cancers and has the potential to be used as a novel biomarker. Int J Clin Exp Pathol. 2010;3:328–37. [PMC free article] [PubMed] [Google Scholar]
- 71.Liu K, Chen C, Guo Y, Lam R, Bian C, et al. Structural basis for recognition of arginine methylated Piwi proteins by the extended Tudor domain. Proc Natl Acad Sci U S A. 2010;107:18398–403. doi: 10.1073/pnas.1013106107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu L, Qi H, Wang J, Lin H. PAPI, a Novel Tudor Domain-Containing Protein, Complexes with AGO3, Me31b and Tral in the Nuage to Regulate Transposon Silencing. Development. 2011;138:1863–73. doi: 10.1242/dev.059287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu X, Sun Y, Guo J, Ma H, Li J, et al. Expression of hiwi gene in human gastric cancer was associated with proliferation of cancer cells. Int J Cancer. 2006;118:1922–9. doi: 10.1002/ijc.21575. [DOI] [PubMed] [Google Scholar]
- 74.Liu Y, Mochizuki K, Gorovsky MA. Histone H3 lysine 9 methylation is required for DNA elimination in developing macronuclei in Tetrahymena. Proc Natl Acad Sci U S A. 2004;101:1679–84. doi: 10.1073/pnas.0305421101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Megosh HB, Cox DN, Campbell C, Lin H. The role of PIWI and the miRNA machinery in Drosophila germline determination. Curr Biol. 2006;16:1884–94. doi: 10.1016/j.cub.2006.08.051. [DOI] [PubMed] [Google Scholar]
- 76.Mochizuki K, Fine NA, Fujisawa T, Gorovsky MA. Analysis of a piwi-related gene implicates small RNAs in genome rearrangement in tetrahymena. Cell. 2002;110:689–99. doi: 10.1016/s0092-8674(02)00909-1. [DOI] [PubMed] [Google Scholar]
- 77.Mochizuki K, Gorovsky MA. A Dicer-like protein in Tetrahymena has distinct functions in genome rearrangement, chromosome segregation, and meiotic prophase. Genes Dev. 2005;19:77–89. doi: 10.1101/gad.1265105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morrish TA, Gilbert N, Myers JS, Vincent BJ, Stamato TD, et al. DNA repair mediated by endonuclease-independent LINE-1 retrotransposition. Nat Genet. 2002;31:159–65. doi: 10.1038/ng898. [DOI] [PubMed] [Google Scholar]
- 79.Moshkovich N, Lei EP. HP1 recruitment in the absence of argonaute proteins in Drosophila. PLoS Genet. 6:e1000880. doi: 10.1371/journal.pgen.1000880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Newmark PA, Reddien PW, Cebria F, Sanchez Alvarado A. Ingestion of bacterially expressed double-stranded RNA inhibits gene expression in planarians. Proc Natl Acad Sci U S A. 2003;100(Suppl 1):11861–5. doi: 10.1073/pnas.1834205100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Newmark PA, Sanchez Alvarado A. Bromodeoxyuridine specifically labels the regenerative stem cells of planarians. Dev Biol. 2000;220:142–53. doi: 10.1006/dbio.2000.9645. [DOI] [PubMed] [Google Scholar]
- 82.Nishida KM, Okada TN, Kawamura T, Mituyama T, Kawamura Y, et al. Functional involvement of Tudor and dPRMT5 in the piRNA processing pathway in Drosophila germlines. Embo J. 2009;28:3820–31. doi: 10.1038/emboj.2009.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Okamura K, Ishizuka A, Siomi H, Siomi MC. Distinct roles for Argonaute proteins in small RNA-directed RNA cleavage pathways. Genes Dev. 2004;18:1655–66. doi: 10.1101/gad.1210204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pal-Bhadra M, Leibovitch BA, Gandhi SG, Rao M, Bhadra U, et al. Heterochromatic silencing and HP1 localization in Drosophila are dependent on the RNAi machinery. Science. 2004;303:669–72. doi: 10.1126/science.1092653. [DOI] [PubMed] [Google Scholar]
- 85.Palakodeti D, Smielewska M, Lu YC, Yeo GW, Graveley BR. The PIWI proteins SMEDWI-2 and SMEDWI-3 are required for stem cell function and piRNA expression in planarians. RNA. 2008;14:1174–86. doi: 10.1261/rna.1085008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pan J, Goodheart M, Chuma S, Nakatsuji N, Page DC, Wang PJ. RNF17, a component of the mammalian germ cell nuage, is essential for spermiogenesis. Development. 2005;132:4029–39. doi: 10.1242/dev.02003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Parker R, Sheth U. P bodies and the control of mRNA translation and degradation. Mol Cell. 2007;25:635–46. doi: 10.1016/j.molcel.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 88.Patil VS, Kai T. Repression of Retroelements in Drosophila Germline via piRNA Pathway by the Tudor Domain Protein Tejas. Curr Biol. 2010 doi: 10.1016/j.cub.2010.02.046. [DOI] [PubMed] [Google Scholar]
- 89.Philippe H, Derelle R, Lopez P, Pick K, Borchiellini C, et al. Phylogenomics revives traditional views on deep animal relationships. Curr Biol. 2009;19:706–12. doi: 10.1016/j.cub.2009.02.052. [DOI] [PubMed] [Google Scholar]
- 90.Pick KS, Philippe H, Schreiber F, Erpenbeck D, Jackson DJ, et al. Improved phylogenomic taxon sampling noticeably affects nonbilaterian relationships. Mol Biol Evol. 2010;27:1983–7. doi: 10.1093/molbev/msq089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ponting CP. Tudor domains in proteins that interact with RNA. Trends Biochem Sci. 1997;22:51–2. doi: 10.1016/s0968-0004(96)30049-2. [DOI] [PubMed] [Google Scholar]
- 92.Putnam NH, Srivastava M, Hellsten U, Dirks B, Chapman J, et al. Sea anemone genome reveals ancestral eumetazoan gene repertoire and genomic organization. Science. 2007;317:86–94. doi: 10.1126/science.1139158. [DOI] [PubMed] [Google Scholar]
- 93.Qi H, Watanabe T, Ku HY, Liu N, Zhong M, Lin H. The Yb Body, a Major Site for Piwi-associated RNA Biogenesis and a Gateway for Piwi Expression and Transport to the Nucleus in Somatic Cells. J Biol Chem. 2010;286:3789–97. doi: 10.1074/jbc.M110.193888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Qiao D, Zeeman AM, Deng W, Looijenga LH, Lin H. Molecular characterization of hiwi, a human member of the piwi gene family whose overexpression is correlated to seminomas. Oncogene. 2002;21:3988–99. doi: 10.1038/sj.onc.1205505. [DOI] [PubMed] [Google Scholar]
- 95.Reddien PW, Oviedo NJ, Jennings JR, Jenkin JC, Sanchez Alvarado A. SMEDWI-2 is a PIWI-like protein that regulates planarian stem cells. Science. 2005;310:1327–30. doi: 10.1126/science.1116110. [DOI] [PubMed] [Google Scholar]
- 96.Rentzsch F, Fritzenwanker JH, Scholz CB, Technau U. FGF signalling controls formation of the apical sensory organ in the cnidarian Nematostella vectensis. Development. 2008;135:1761–9. doi: 10.1242/dev.020784. [DOI] [PubMed] [Google Scholar]
- 97.Reuter M, Chuma S, Tanaka T, Franz T, Stark A, Pillai RS. Loss of the Mili-interacting Tudor domain-containing protein-1 activates transposons and alters the Mili-associated small RNA profile. Nat Struct Mol Biol. 2009;16:639–46. doi: 10.1038/nsmb.1615. [DOI] [PubMed] [Google Scholar]
- 98.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 99.Rinkevich Y, Rosner A, Rabinowitz C, Lapidot Z, Moiseeva E, Rinkevich B. Piwi positive cells that line the vasculature epithelium, underlie whole body regeneration in a basal chordate. Dev Biol. 2010;345:94–104. doi: 10.1016/j.ydbio.2010.05.500. [DOI] [PubMed] [Google Scholar]
- 100.Robine N, Lau NC, Balla S, Jin Z, Okamura K, et al. A broadly conserved pathway generates 3′UTR-directed primary piRNAs. Curr Biol. 2009;19:2066–76. doi: 10.1016/j.cub.2009.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rouget C, Papin C, Boureux A, Meunier AC, Franco B, et al. Maternal mRNA deadenylation and decay by the piRNA pathway in the early Drosophila embryo. Nature. 2010;467:1128–32. doi: 10.1038/nature09465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rudin CM, Thompson CB. Transcriptional activation of short interspersed elements by DNA-damaging agents. Genes Chromosomes Cancer. 2001;30:64–71. [PubMed] [Google Scholar]
- 103.Saffman EE, Lasko P. Germline development in vertebrates and invertebrates. Cell Mol Life Sci. 1999;55:1141–63. doi: 10.1007/s000180050363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Saito K, Ishizu H, Komai M, Kotani H, Kawamura Y, et al. Roles for the Yb body components Armitage and Yb in primary piRNA biogenesis in Drosophila. Genes Dev. 2010;24:2493–8. doi: 10.1101/gad.1989510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Saito K, Nishida KM, Mori T, Kawamura Y, Miyoshi K, et al. Specific association of Piwi with rasiRNAs derived from retrotransposon and heterochromatic regions in the Drosophila genome. Genes Dev. 2006;20:2214–22. doi: 10.1101/gad.1454806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Saito K, Siomi MC. Small RNA-mediated quiescence of transposable elements in animals. Dev Cell. 2010;19:687–97. doi: 10.1016/j.devcel.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 107.Schupbach T, Wieschaus E. Female sterile mutations on the second chromosome of Drosophila melanogaster. II. Mutations blocking oogenesis or altering egg morphology. Genetics. 1991;129:1119–36. doi: 10.1093/genetics/129.4.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Seipel K, Yanze N, Schmid V. The germ line and somatic stem cell gene Cniwi in the jellyfish Podocoryne carnea. Int J Dev Biol. 2004;48:1–7. doi: 10.1387/ijdb.15005568. [DOI] [PubMed] [Google Scholar]
- 109.Sharma AK, Nelson MC, Brandt JE, Wessman M, Mahmud N, et al. Human CD34(+) stem cells express the hiwi gene, a human homologue of the Drosophila gene piwi. Blood. 2001;97:426–34. doi: 10.1182/blood.v97.2.426. [DOI] [PubMed] [Google Scholar]
- 110.Shoji M, Tanaka T, Hosokawa M, Reuter M, Stark A, et al. The TDRD9-MIWI2 complex is essential for piRNA-mediated retrotransposon silencing in the mouse male germline. Dev Cell. 2009;17:775–87. doi: 10.1016/j.devcel.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 111.Song JJ, Smith SK, Hannon GJ, Joshua-Tor L. Crystal structure of Argonaute and its implications for RISC slicer activity. Science. 2004;305:1434–7. doi: 10.1126/science.1102514. [DOI] [PubMed] [Google Scholar]
- 112.Specchia V, Piacentini L, Tritto P, Fanti L, D’Alessandro R, et al. Hsp90 prevents phenotypic variation by suppressing the mutagenic activity of transposons. Nature. 2010;463:662–5. doi: 10.1038/nature08739. [DOI] [PubMed] [Google Scholar]
- 113.Surani MA. Reprogramming of genome function through epigenetic inheritance. Nature. 2001;414:122–8. doi: 10.1038/35102186. [DOI] [PubMed] [Google Scholar]
- 114.Taubert H, Greither T, Kaushal D, Wurl P, Bache M, et al. Expression of the stem cell self-renewal gene Hiwi and risk of tumour-related death in patients with soft-tissue sarcoma. Oncogene. 2007;26:1098–100. doi: 10.1038/sj.onc.1209880. [DOI] [PubMed] [Google Scholar]
- 115.Thomson T, Lasko P. Drosophila tudor is essential for polar granule assembly and pole cell specification, but not for posterior patterning. Genesis. 2004;40:164–70. doi: 10.1002/gene.20079. [DOI] [PubMed] [Google Scholar]
- 116.Thomson T, Lin H. The biogenesis and function of PIWI proteins and piRNAs: progress and prospect. Annu Rev Cell Dev Biol. 2009;25:355–76. doi: 10.1146/annurev.cellbio.24.110707.175327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Unhavaithaya Y, Hao Y, Beyret E, Yin H, Kuramochi-Miyagawa S, et al. MILI, a PIWI-interacting RNA-binding Protein, Is Required for Germ Line Stem Cell Self-renewal and Appears to Positively Regulate Translation. J Biol Chem. 2009;284:6507–19. doi: 10.1074/jbc.M809104200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vagin VV, Sigova A, Li C, Seitz H, Gvozdev V, Zamore PD. A distinct small RNA pathway silences selfish genetic elements in the germline. Science. 2006;313:320–4. doi: 10.1126/science.1129333. [DOI] [PubMed] [Google Scholar]
- 119.Vagin VV, Wohlschlegel J, Qu J, Jonsson Z, Huang X, et al. Proteomic analysis of murine Piwi proteins reveals a role for arginine methylation in specifying interaction with Tudor family members. Genes Dev. 2009;23:1749–62. doi: 10.1101/gad.1814809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vasileva A, Tiedau D, Firooznia A, Muller-Reichert T, Jessberger R. Tdrd6 is required for spermiogenesis, chromatoid body architecture, and regulation of miRNA expression. Curr Biol. 2009;19:630–9. doi: 10.1016/j.cub.2009.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Verdel A, Jia S, Gerber S, Sugiyama T, Gygi S, et al. RNAi-mediated targeting of heterochromatin by the RITS complex. Science. 2004;303:672–6. doi: 10.1126/science.1093686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Volpe TA, Kidner C, Hall IM, Teng G, Grewal SI, Martienssen RA. Regulation of heterochromatic silencing and histone H3 lysine-9 methylation by RNAi. Science. 2002;297:1833–7. doi: 10.1126/science.1074973. [DOI] [PubMed] [Google Scholar]
- 123.Wang G, Reinke V. A C. elegans Piwi, PRG-1, regulates 21U-RNAs during spermatogenesis. Curr Biol. 2008;18:861–7. doi: 10.1016/j.cub.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang J, Saxe JP, Tanaka T, Chuma S, Lin H. Mili interacts with tudor domain-containing protein 1 in regulating spermatogenesis. Curr Biol. 2009;19:640–4. doi: 10.1016/j.cub.2009.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Watanabe T, Takeda A, Tsukiyama T, Mise K, Okuno T, et al. Identification and characterization of two novel classes of small RNAs in the mouse germline: retrotransposon-derived siRNAs in oocytes and germline small RNAs in testes. Genes Dev. 2006;20:1732–43. doi: 10.1101/gad.1425706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wilson JE, Connell JE, Macdonald PM. aubergine enhances oskar translation in the Drosophila ovary. Development. 1996;122:1631–9. doi: 10.1242/dev.122.5.1631. [DOI] [PubMed] [Google Scholar]
- 127.Wittlieb J, Khalturin K, Lohmann JU, Anton-Erxleben F, Bosch TC. Transgenic Hydra allow in vivo tracking of individual stem cells during morphogenesis. Proc Natl Acad Sci U S A. 2006;103:6208–11. doi: 10.1073/pnas.0510163103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wu L, Belasco JG. Let me count the ways: mechanisms of gene regulation by miRNAs and siRNAs. Mol Cell. 2008;29:1–7. doi: 10.1016/j.molcel.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 129.Yin H, Lin H. An epigenetic activation role of Piwi and a Piwi-associated piRNA in Drosophila melanogaster. Nature. 2007;450:304–8. doi: 10.1038/nature06263. [DOI] [PubMed] [Google Scholar]