Table 1.
Synthesis and alkyne C(sp) 13C NMR chemical shifts of gold π-alkyne complexes 1a–1e and 2a–2c.
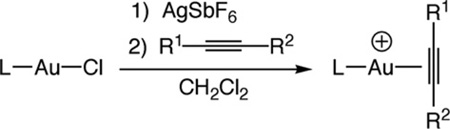 | |||||
|---|---|---|---|---|---|
| entry | L | alkyne | complex | yield (%)a | δ C=C |
| 1 | P(t-Bu)2(o-biphenyl) | 1a | 98 | 91.4 | |
| 2 | P(t-Bu)2(o-biphenyl) | 1b | 96 | 88.7, 75.4 | |
| 3 | P(t-Bu)2(o-biphenyl) | 1c | 90 | 98.3, 87.0 | |
| 4 | P(t-Bu)2(o-biphenyl) | 1d | 91 | 84.9 | |
| 5 | P(t-Bu)2(o-biphenyl) | 1e | (92) | 90.6, 86.0 | |
| 6 | IPr | 2a | 94 | 87.2 | |
| 7 | IPr | 2b | 99 | 86.9, 83.5 | |
| 8 | IPr | 2c | (97) | 81.5 | |
Isolated yield. Values in parentheses refers to 1H NMR yield for in situ generated complexes.
