Fig. 3.
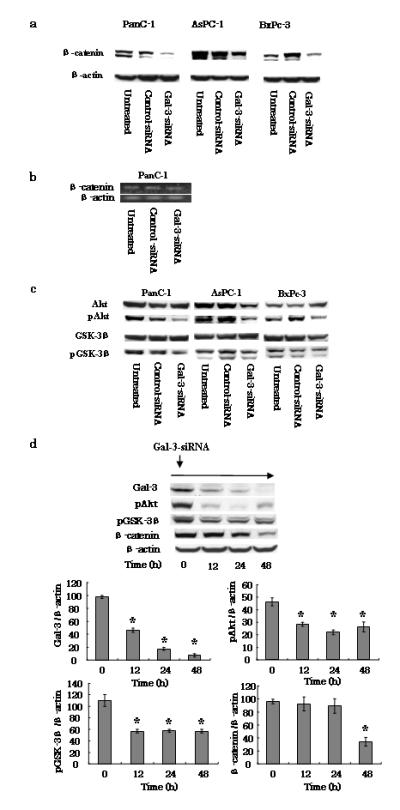
Effects of gal-3 silencing on the β-catenin/Wnt pathway. a Expression of β-catenin in gal-3-silenced cells. After transfection of cells with either control- or gal-3-siRNA for 48 h (or cells were left untreated), the cells were harvested and maintained for 72 h with DMEM containing 10% FBS. Total cell lysates were isolated and β-catenin expression levels were detected by western blotting using an anti-human β-catenin antibody. β-actin was used as a loading control. b Representative RT-PCR amplification of β-catenin. PanC-1 cells transfected with either control- or gal-3-siRNA using the same conditions as described above (a) were harvested, total RNA was extracted, and the β-catenin and β-actin (control) mRNA levels were detected by RT-PCR. c Effect of gal-3 silencing on expression of Akt and GSK-3β. Total and phosphorylated (p) Akt and GSK-3β expression levels in control- or gal-3-siRNA-transfected cells were detected by western blotting. d PanC-1 cells transfected with gal-3-siRNA and harvested at 0, 12, 24, and 48 h. Western blotting revealed time-dependent changes in the phosphorylation of both Akt and GSK-3β, and in β-catenin, following gal-3 silencing. Densitometry (mean ± SD) revealed significant decreases in the levels of these proteins in gal-3-siRNA-transfected cells (n = 3). Data of densitometry were analyzed by using ANOVA. Significant differences were detected in gal-3, pAkt, pGSK-3β and β-catenin expression levels. Dunnett’s tests were used to assess the differences in these expression levels compared with 0 hour. *P < 0.05 versus 0 h.
