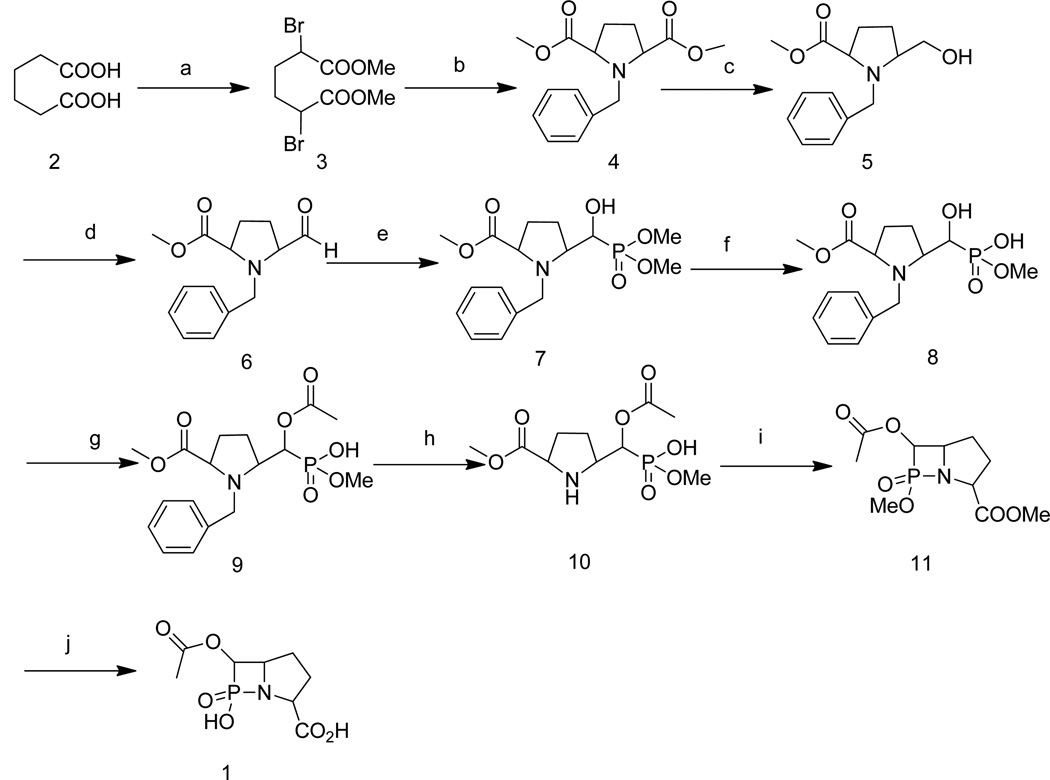
Scheme 1.
Synthetic route of β-phospholactam 1: a: (1) acetyl chloride, (2) Br2, (3) MeOH; b: benzylamine, K2CO3, CH3CN; c: NaBH4, EtOH; d: oxalyl chloride, DMSO, dichloromethane, −78 °C; e: dimethyl phosphonate, 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), anhydrous THF; f: NaI, acetone; g: acetic anhydride, pyridine; h: Pd-C, H2; i: NaH, 15-crown-5, dichloromethane; j: LiOH, H2O, MeOH.