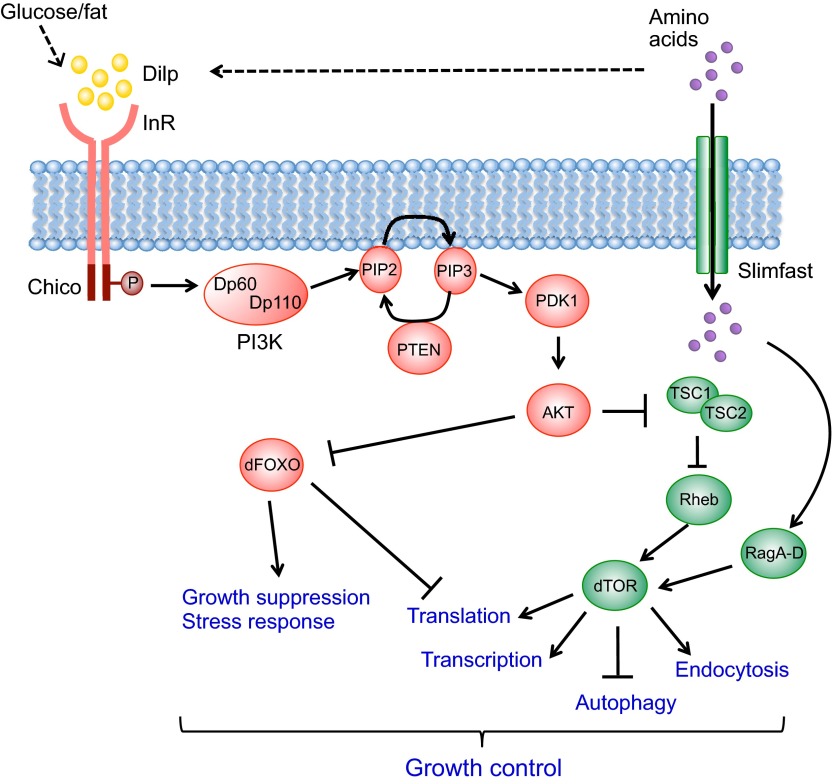
Fig. 1.
The Insulin receptor pathway in Drosophila. The presence of sugars, fat and amino acids triggers the secretion of Drosophila insulin-like peptides (Dilps; insulin or IGF in mammals) from brain neuroendocrine cells. Dilp is recognized by Insulin receptor (InR; insulin receptor or IGFR in mammals) in the peripheral tissues and phosphorylates its substrate Chico (IRS1-4 in mammals). The downstream phosphorylation cascade includes the Dp60/Dp110 (Pi3K21B/Pi3K92E) complex (PI3K), PTEN, PDK1 and AKT, and controls the nuclear localization of dFOXO. In addition to the canonical InR/AKT/dFOXO pathway, AKT represses the TSC1/TSC2 complex, causing activation of Rheb and dTOR. Additionally, amino acids are imported into the cell by Slimfast (SLC7A family) and regulate dTOR via the RagA-D GTPase proteins. The modulation of the InR/dTOR pathways affects multiple cellular processes and thus links nutrient availability to growth control. The dashed arrows indicate indirect systemic controls of Dilp secretion through the fat body-derived Factor X or Upd2 (see also Fig. 6).