Fig. 4.
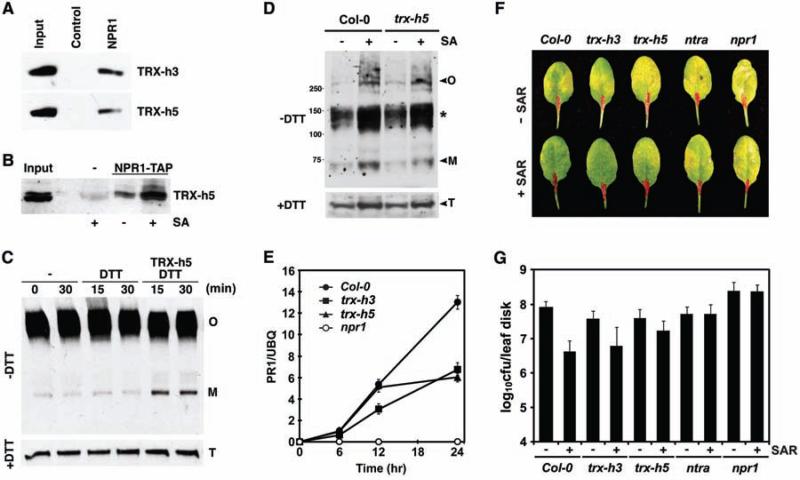
TRX is a redox mediator of NPR1. (A) Immobilized His6-NH pulls down TRXs in vitro. TRXs were detected with protein-specific antibodies. (B) 35S::NPR1-TAP transformed and untransformed plants were treated with or without SA. SA treatment enhanced coimmunoprecipitation of TRX-h5 with NPR1-TAP as detected by an antibody to TRX-h5. (C) NPR1-GFP oligomer (O) is reduced to monomer (M) by TRX-h5 in vitro. DTT (0.33 mM) was added to recycle TRX activity. Detection of total (T) NPR1-GFP verified equal loading. (D) In vivo reduction of NPR1 oligomer to monomer in Col-0 and trx-h5 plants 12 hours after SA treatment. Non-reducing (–DTT) and reducing (+DTT) SDS-polyacrylamide gel electrophoresis were performed in parallel, and the endogenous NPR1 was detected with an antibody to NPR1. The asterisk indicates possible NPR1 dimer complexes. (E) SA-induced PR-1 gene expression is compromised in trx mutants. PR-1 expression was determined by real-time PCR and normalized with ubiquitin (UBQ). Error bars represent SD. (F and G) Induction of SAR significantly decreased disease symptoms and Psm ES4326 growth in Col-0 plants but not in the trx, ntra, and npr1-1 mutants. Error bars represent 95% confidence limits (n = 8 samples).