Abstract
Background
Little information exists regarding the prevalence and natural history of pericardial disease in patients with leukemia. Recently, it has been reported that the use of histone deacytelase inhibitors (HDACi) is associated with an increased incidence of pericardial effusions (PEfs). To study the characteristics and treatment relationships of PEfs in patients with leukemia, we retrospectively analyzed a cohort of patients with leukemia evaluated at a single center.
Methods
We reviewed 2592 patients with acute myeloid leukemia (AML, N= 1282, 49%), acute lymphocytic leukemia (ALL, N= 336, 13%), or myelodysplastic syndrome (MDS, N=974, 38%), who were evaluated from 8/2003 to 7/2008. Electronic medical records were reviewed to select patients that had underwent at least one echocardiographic evaluation. Data regarding diagnosis, timing, effusion size, survival, and prior therapy was collected in the patients that had echocardiographic evidence of PEfs.
Results
PEfs were detected in 325 (20%) of the patients who had echocardiograms: 21% in AML, 23% in ALL, and 18% in MDS patients. Only a small portion of PEfs were detected prior to the initiation of therapy: 26% in AML, 25% ALL, and 15% in MDS. Most PEfs were of minimal size (70%) overall. No significant differences in effusion characteristics, including severity, were observed among different types of therapies. The presence of PEfs had no impact on the survival of patients evaluated.
Conclusions
PEfs are relatively common in patients with leukemia and do not appear to be related to specific types of therapy or to survival.
Keywords: leukemia, pericardial effusions, histone deacetylase inhibitors
INTRODUCTION
Leukemia is a systemic disorder and can frequently involve the hepatic, renal, circulatory, and cardiopulmonary systems1. However, little is known about pericardial disease in patients with leukemia.
Pericardial effusions (PEfs) have been reported to be rare at initial diagnosis in leukemia2. However, PEfs have long been viewed as part of the leukemic disease process, arising secondary to hemorrhagic, infectious, or leukemic infiltrates3. PEfs have also been thought to be associated with certain forms of chemotherapy including bleomycin, all-trans retinoic acid, and cyclophosphamide4. PEfs are a potentially life threatening condition especially if cardiac tamponade or constrictive pericarditis develops. Echocardiography is the safest and most commonly used modality for diagnosing PEfs5.
Our interest in PEfs developed as the result of a report of an increased incidence of PEfs in patients treated with histone deacetylase inhibitor (HDACi), MGCD0103 (unpublished information). MGCD0103 is a novel small molecule that is being studied as a single-agent or in combination chemotherapy regimens for solid tumors6, lymphoma7, and leukemia8. This report of increased PEfs was performed by the sponsor of a clinical trial of MGCD0103 in combination with 5-azacytidine (Celgene Corporation) that resulted in early termination of this study.
Although QT interval prolongation has been reported with the use of HDACi, no other significant cardiac toxicities have been reported with the use of HDACi9. Therefore, the identification of a new class specific toxicity could have significant implications for the further development of these agents. This lead to the need for the current study of PEfs in leukemia.
In the present study, we sought to determine the rate, severity, and timing of PEfs in leukemia. Furthermore, we sought to investigate if any specific class of chemotherapeutic agents, including HDACis, were associated with more severe PEfs.
PATIENTS AND METHODS
We reviewed the electronic medical records of 2592 patients evaluated in the Leukemia Department at MD Anderson Cancer Center (MDACC) between August 2003 and July 2008 following institutional guidelines. These patients were diagnosed with acute myelogenous leukemia (AML: N= 1282, 49%), acute lymphocytic leukemia (ALL: N= 336, 13%), or myelodysplastic syndrome (MDS: N=974, 38%). Of the initial cohort, 1600 patients (62%) had at least one echocardiogram performed at some point during their treatment course.
The electronic medical record for each patient with a PEf was reviewed for all the treatment history, leukemia characteristics, survival, PEf size, and evolution of the PEfs. A numerical score was assigned to grade the size of the PEf based on the echocardiogram report: minimal (1), small (2), moderate (3), large (4), and not classifiable (0). Five patients (1.5%) had complete records but the PEf size was indeterminate and could not be classified (grade 0). For patients with multiple echocardiograms, data at the time of the first echocardiogram revealing a PEf was used in our analysis. Subsequent echocardiograms were also reviewed to follow the evolution of PEfs sequentially. Specifically, we collected data from the next echocardiogram after the initial PEf was detected and also the last echocardiogram available for the patient. Only those patients with followup echocardiograms were included in this analysis. The determination of other concomitant systemic effusions such as pleural effusions and ascites was made by imaging studies such as a chest radiograph, CT scan, or an abdominal ultrasound.
Survival was calculated from the initial presentation to MDACC. Survival duration was plotted according to the Kaplan and Meier method.
RESULTS
Patient Characteristics
A total of 2592 patients charts were reviewed with 1600 patients (62%) having an echocardiogram at some point during the treatment course. Patient characteristics are shown in Table 1. Patients that had an echocardiogram were significantly younger than those who did not. (Table 1).
Table 1.
Patient Characteristics.
| Echo | % Pts | No Echo |
% Pts | p- value |
Total | %Pts | |
|---|---|---|---|---|---|---|---|
| Male | 911 | 56.9 | 624 | 62.9 | 0.003 | 1535 | 59.2 |
| Female | 689 | 43.1 | 368 | 37.1 | 1057 | 40.8 | |
| ALL | 301 | 18.8 | 35 | 3.5 | <0.001 | 336 | 13 |
| AML | 894 | 55.9 | 388 | 39.1 | 1282 | 49.5 | |
| MDS | 405 | 25.3 | 569 | 57.4 | 974 | 37.6 | |
| Total | 1600 | 992 | 2592 | ||||
| Age | 56 (14- 92) |
65 (16- 93) |
<0.001 | 59.5 (14- 93) |
Frequency and Size of PEfs in Leukemia
Overall, a PEf was detected in 325 patients (20%) of the 1600 patients evaluated: 21% in AML (n = 185), 23% in ALL (n = 68), and 18% in MDS (n = 72) patients (P = 0.27). In the 325 patients with evidence of PEfs, most effusions occurred after receiving some form of therapy: 74% in AML (n = 136), 75% in ALL (n = 51), and 85% in MDS (n = 61) patients (P = 0.20). The remainder of PEfs were present before therapy or at initial presentation (Figure 1). The distribution of PEfs before therapy accounted for 26% in AML (n = 49), 25% in ALL (n = 17), and 15% in MDS (n = 11) patients (P = 0.20).
Figure 1. Distribution of Pericardial Effusions by Timing of Occurrence.
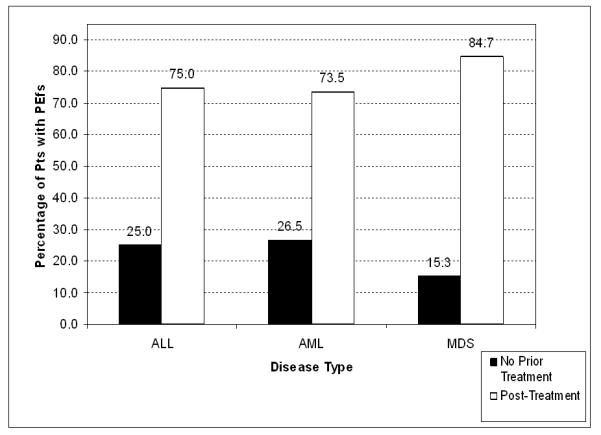
Percentage of patients with leukemia with PEfs prior to any therapy (black column) or after therapy (white column). ALL, acute lymphocytic leukemia; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome.
The majority of the PEfs in leukemia were of minimal or trace size (grade 1, 70%). There was a similar overall distribution of PEf sizes or grades in all three leukemias (P = 0.85; Figure 2A). The size of PEfs were not significantly different for the three leukemias prior to therapy or after therapy (P = 0.77, pre-treatment and P = 0.75, post-treatment; Figures 2B and 2C). Less than 9% of PEfs in ALL, AML, or MDS were considered to be moderate or large in size (grade 3 or 4) at any time point. The initial PEf detected was on average the largest one (Figure 3). The follow-up echocardiogram revealed a decrease in size across all disease categories. In patients with more than one followup echocardiogram, the last echo revealed a slight decrease in PEf size, except in MDS.
Figure 2. Distribution of Pericardial Effusions by Size and Disease Type.
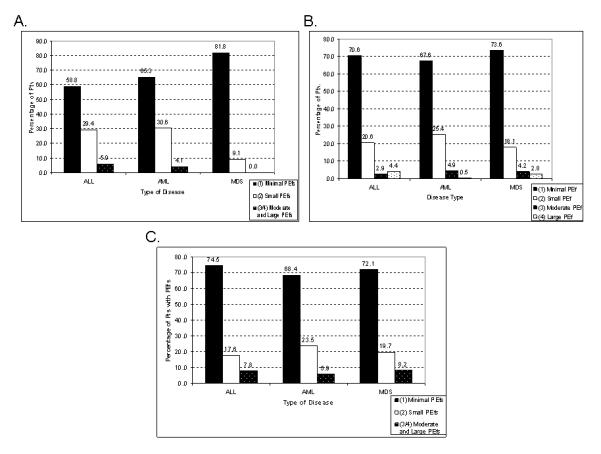
Distribution and size of PEfs in patients with leukemia. A. Overall. B. Prior to any therapy. C. After therapy. In the Y-axis, percent of patients. ALL, acute lymphocytic leukemia; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome.
Figure 3. Pericardial Effusion Evolution by Average Size.
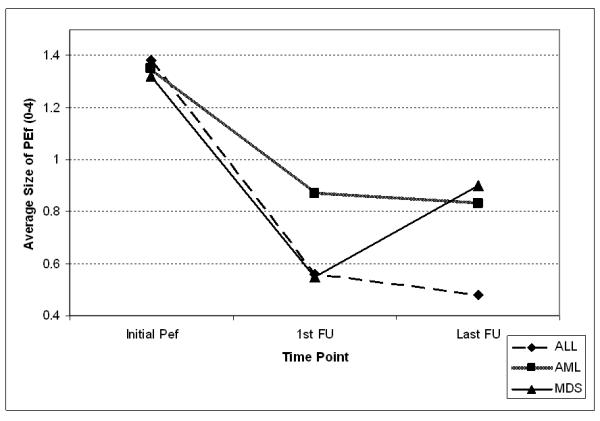
Evolution in terms of size of PEfs. In the Y-axis, average size of effusion by grade 0 to 4. PEf, pericardial effusion; 1st FU, first follow; Last FU, last follow up
Effect of Therapy on PEf development
We subsequently studied the frequency and size of PEfs in relation to a specific class or type of treatment. Those patients who had received no prior therapy with the same diagnosis were used as controls. We compared the distribution of the size of PEfs in patients who had a prior exposure to a particular class of chemotherapeutic agents to the control group.
No single type of therapy, including HDACi, was found to correlate with larger sized PEfs with any statistical significance (Table 2). The distribution of PEfs in the group of patients treated with HDACi does not suggest more severe effusions in this group compared with the others.
Table 2.
Incidence of large PEfs Divided by Exposure to Chemotherapeutic Class
| Disease | Treatment/no rx |
Total
Pts |
>=2 | p (indiv. Rx vs no rx) |
overall p |
>=3 | p (indiv. Rx vs no rx) |
overall p |
|---|---|---|---|---|---|---|---|---|
| AML | Anthracyclines combination |
86 | 24 | 0.44 | 4 | 1 | ||
| HDAC inhibitors | 18 | 8 | 0.57 | 2 | 0.29 | |||
| Hypomethylating Agents | 35 | 11 | 0.82 | 3 | 0.64 | |||
| Monocolonal Antibodies | 30 | 8 | 0.62 | 1 | 1 | |||
| Akylating Agents | 49 | 18 | 1 | 0.83 | 2 | 1 | 0.67 | |
| Antimetabolites | 117 | 36 | 0.72 | 8 | 0.72 | |||
| TKIs | 11 | 4 | 1 | 1 | 0.46 | |||
| Topoisomerase Inhibitors | 15 | 8 | 0.23 | 3 | 0.08 | |||
| ATRA | 10 | 3 | 1 | 1 | 0.43 | |||
| Arsenic Trioxide | 8 | 2 | 0.7 | 1 | 0.37 | |||
| Prior Stem Cell Transplant |
16 | 4 | 0.55 | 0 | 1 | |||
| Other Chemotherapy | 28 | 11 | 0.81 | 2 | 0.62 | |||
| No Treatment | 49 | 17 | 2 | |||||
| ALL | Anthracyclines | 51 | 13 | 0.53 | 4 | 1 | ||
| Monoclonal Antibodies | 16 | 4 | 0.71 | 0 | 1 | |||
| Alkylating Agents | 49 | 12 | 0.53 | 4 | 1 | |||
| Antimetabolites | 47 | 12 | 0.53 | 0.72 | 4 | 1 | 0.84 | |
| TKIs | 13 | 5 | 1 | 1 | 1 | |||
| Aparaginase | 14 | 2 | 0.24 | 0 | 1 | |||
| Vinca alkaloids | 51 | 13 | 0.53 | 4 | 1 | |||
| Prior Stem Cell Transplant |
5 | 2 | 1 | 1 | 0.41 | |||
| Other Chemotherapy | 5 | 3 | 0.61 | 1 | 0.41 | |||
| No Treatment | 17 | 6 | 1 | |||||
| MDS | Anthracyclines | 21 | 9 | 0.1 | 3 | 0.53 | ||
| HDAC inhibitors | 8 | 2 | 0.54 | 1 | 0.42 | |||
| Hypomethylating Agents | 33 | 10 | 0.24 | 4 | 0.56 | |||
| Monoclonal Antibodies | 7 | 1 | 1 | 0.35 | 0 | n/a | 0.82 | |
| Alkylating Agents | 22 | 7 | 0.22 | 2 | 0.54 | |||
| Antimetabolites | 31 | 7 | 0.66 | 2 | 1 | |||
| Topoisomerase Inhibitors | 7 | 0 | 1 | 0 | n/a | |||
| Prior Stem Cell Transplant |
9 | 2 | 0.56 | 0 | n/a | |||
| Other Chemotherapy | 20 | 8 | 0.11 | 2 | 0.53 | |||
| No Treatment | 11 | 1 | 0 |
Relationship with Ascites or Pleural Effusions
Of the 325 of patients with PEfs, most were often found to have evidence of other systemic effusions. Approximately 23% of patients (n = 75) were found to have ascites at the time of a PEf based on abdominal imaging studies. Approximately 75% of patients (n = 244) had a pleural effusion based on chest imaging studies. Concurrent ascites, pleural effusion, and a PEf were found in 23% of the patients (n = 75).
Clinical Management of PEfs
Only 10 patients (3%) required pericardiocentesis due to tamponade at the time of the initial PEf. Most of the PEfs were either asymptomatic or did not require direct intervention. Of interest, analysis of the pericardial fluid revealed that 4 patients (40%) had leukemic blasts present. Analysis of the other PEfs revealed hemorrhagic infiltrates or serosanguinous fluid.
Impact on Survival
We also studied the impact of having a PEf on survival. Both patients with or without a PEf had a median survival of 60 weeks (P = 0.63, Figure 4A). Patients who had an echocardiogram had a median survival of 60 weeks versus 53 weeks for those without an echocardiogram (P = 0.38, Figure 4B).
Figure 4. Patient survival.
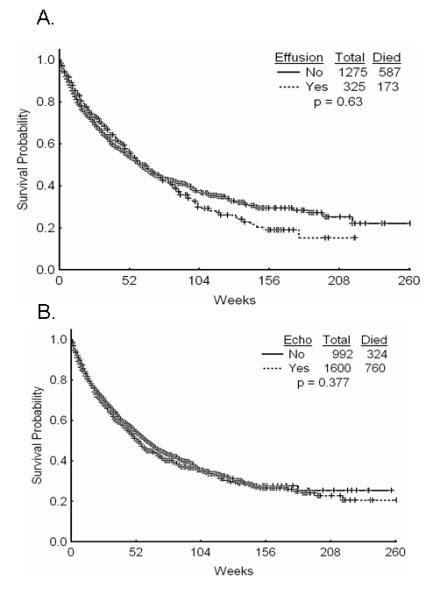
A. Overall survival of patients with or without effusion. B. Overall survival of patients with or without and achocardiogram.
DISCUSSION
Leukemias are systemic disorders that can clinically effect any organ system. Organ dysfunction is not uncommon particularly at the initial presentation of patients with acute leukemias. Renal, hepatic, and cardiopulmonary impairment and infections frequently preclude initiation of optimal chemotherapy in a significant portion of patients.
Although cardiopulmonary complications including pericardial effusions are observed in the course of treating patients with leukemia, little is known specifically about the frequency and severity and clinical implications of PEfs in leukemia. Interest in this issue developed from the recent report of an increased incidence of PEfs in patients treated with the HDACi, MGCD0103. This observation has lead to the halting of all clinical trials using this compound until further evaluation is completed as determined by the sponsors (Celgene and Methlygene) and the US Food and Drug Administration (FDA). Because of the potential importance and promise of HDACi in the treatment of leukemias, we were compelled to examine the incidence, therapeutic relationships, and clinical significance of PEfs in leukemia.
To study PEfs in leukemia, we identified 2592 patients with ALL, AML, or MDS seen at MDACC from 2003 to 2008 (Table 1). Patients that had echocardiograms versus those who did not were similar in characteristics except younger average age. The difference in age is likely related to the more frequent use of anthracycline based therapy during induction therapy in younger patients (<60 years). The incidence of a PEfs was similar between ALL, AML, and MDS (15-27%) at initial presentation and prior to any leukemia treatments (Figure 1). The majority of PEfs in all three diseases occurred after systemic treatment had been initiated.
Overall, most of the PEfs (70%) were clinically insignificant and were considered to be trivial or minimal by echocardiographic measurements. Only a small portion of PEfs (ranging from 5 to 7%) were considered moderate or large (Figure 2A). The sizes of PEfs were similar whether discovered prior to therapy or after (Figure 2B and 2C). We also studied the evolution of PEf size over time. This analysis indicated that the largest PEf was the initial effusion detected. Overall, PEfs decrease in size with time and continued therapy but rarely resolved completely (Figure 3).
Since PEfs were clearly more frequent in patients receiving therapy, we studied relationships between PEf severity and chemotherapeutic classes. Table 2 shows how frequently moderate sized or larger pericardial effusions (grades >=2 or >=3) were associated with exposure to drugs from various chemotherapeutic classes. No one class appears to be significantly linked with larger PEfs.
We also sought to determine the clinical significance of having an echocardiogram or a PEf in leukemia patients. Survival does not appear to be different between those patients with a PEf and those without (Figure 4A). Similarly, survival is not significantly different for patients who had an echocardiogram versus those who did not (Figure 4B). This suggests that PEfs have very little effect on the outcome in patients with leukemia. The increased frequency of PEfs during therapy is likely clinically insignificant as well.
There are several limitations with our analysis. Most are related to the retrospective nature of this study and the heterogeneity of the patient characteristics and the types of therapies used. The first question relates to why only a portion of patients had an echocardiogram (Figure 1). Most likely this is a result of inclusion in clinical trials or the use of known cardiotoxic therapies such as anthracyclines. This also explains the younger age of the patients undergoing an echocardiogram. Patients who did have an echocardiogram were on average 9 years younger than those who did not. When gathering data on the evolution of PEfs, we are limited by the number of follow-up studies. Many patients had multiple follow-up echocardiograms whereas others did not have any. This poses a problem because the lack of follow-up studies could be due to a variety of reasons including an improving clinical picture or death.
Another major limitation is that we cannot provide data in most cases of the specific type of the PEfs. Only 3% of the patients with PEfs had a pericardiocentesis. However, most patients with PEfs also had other systemic effusions. Twenty-four percent had evidence of ascites on abdominal imaging and 77% had evidence of pleural effusions on chest imaging studies. This supports the idea that PEfs are related to a systemic process, either inflammatory, infectious, or from the leukemia itself. This would also strengthen the notion that PEfs are by and large part of the natural history of the disease. Finally, although having an echocardiogram was not associated with distinct prognosis, this should not be used as a reason not to perform echocardiographic evaluation in patients receiving therapy for leukemia.
In summary, most PEfs occur in leukemia patients after some therapy. However no chemotherapeutic class appears to be directly related to causing more severe PEfs. Most PEfs are insignificant in size and appear to have little effect on survival in patients with leukemia.
Condensed abstract.
Little is known in terms of the characteristics of pericardial effusions (PEfs) in leukemia. Recently, it has been suggested that therapy with histone deacetylase inhibitors increases the incidence of PEfs. Here we show that PEfs are relatively common in leukemia, but are not related to specific types of therapy and have no impact on the survival of patients with leukemia.
Acknowledgements
Supported in part by the MDS P01 grant 5P01CA108631-04
Footnotes
Authorship: KS analyzed and collected the data and wrote the manuscript; VG-G and AR collected data; SP analyzed and collected data; JC and HK contributed patients to the study; GG-M designed the study, analyzed data, and wrote the manuscript.
REFERENCES
- 1.Roberts WC, Bodey GP, Wertlake PT. The heart in acute leukemia. A study of 420 autopsy cases. Am J Cardiol. 1968;21:388–412. doi: 10.1016/0002-9149(68)90143-4. [DOI] [PubMed] [Google Scholar]
- 2.Arya LS, Narain S, Thavaraj V, Saxena A, Bhargava M. Leukemic pericardial effusion causing cardiac tamponade. Med Pediatr Oncol. 2002;38:282–4. doi: 10.1002/mpo.1327. [DOI] [PubMed] [Google Scholar]
- 3.Bierman HR, Perkins EK, Ortega P. Pericarditis in patients with leukemia. Am Heart J. 1952;43:413–22. doi: 10.1016/0002-8703(52)90084-7. [DOI] [PubMed] [Google Scholar]
- 4.Floyd JD, Nguyen DT, Lobins RL, Bashir Q, Doll DC, Perry MC. Cardiotoxicity of cancer therapy. J Clin Oncol. 2005;23:7685–96. doi: 10.1200/JCO.2005.08.789. [DOI] [PubMed] [Google Scholar]
- 5.Troughton RW, Asher CR, Klein AL. Pericarditis. Lancet. 2004;363:717–27. doi: 10.1016/S0140-6736(04)15648-1. [DOI] [PubMed] [Google Scholar]
- 6.Siu LL, Pili R, Duran I, Messersmith WA, Chen EX, Sullivan R, et al. Phase I study of MGCD0103 given as a three-times-per-week oral dose in patients with advanced solid tumors. J Clin Oncol. 2008;26:1940–7. doi: 10.1200/JCO.2007.14.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crump M, CA, Assouline S, Rizzieri D, Wedgwood A, McLaughlin P, Laille E, Li Z, Martell RE, Younes A. Treatment of relapsed or refractory non-hodgkin lymphoma with the oral isotype-selective histone deacetylase inhibitor MGCD0103: Interim results from a phase II study. J. Clin Oncol. 2008;26 abstr 8528. [Google Scholar]
- 8.Le Tourneau C, Siu LL. Promising antitumor activity with MGCD0103, a novel isotype-selective histone deacetylase inhibitor. Expert Opin Investig Drugs. 2008;17:1247–54. doi: 10.1517/13543784.17.8.1247. [DOI] [PubMed] [Google Scholar]
- 9.Rasheed W, Bishton M, Johnstone RW, Prince HM. Histone deacetylase inhibitors in lymphoma and solid malignancies. Expert Rev Anticancer Ther. 2008;8:413–32. doi: 10.1586/14737140.8.3.413. [DOI] [PubMed] [Google Scholar]


