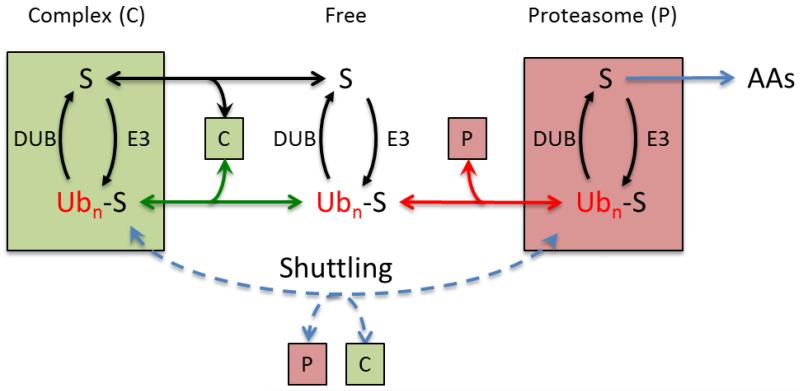
Figure 1.
A given substrate (S) can exist in the free state or bound in a complex (C), each location containing its own distinct E3 and DUB. The strength of binding can be different for the free (black line) or ubiquitinated (green line) substrate. Depending on the value of these binding constants, ubiquitination can either recruit or release S from C. Ubiquitinated protein can be directly bound by the proteasome (red line) or shuttled to the proteasome (dashed blue line) if C is a ubiquitin receptor.