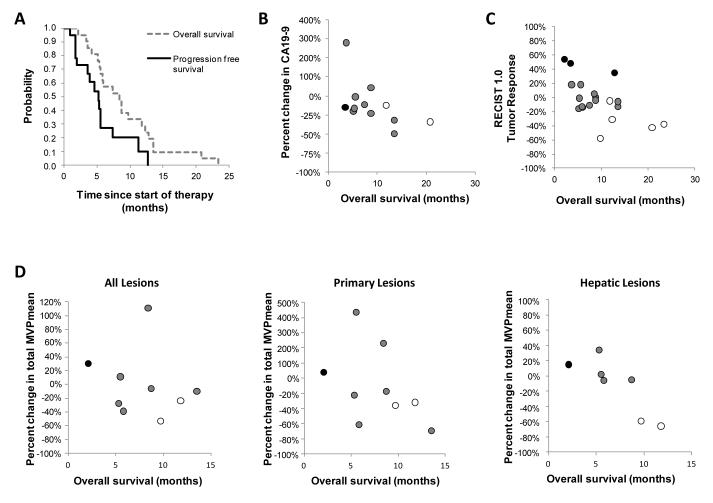
Figure 4.
Biological correlatives for overall survival after treatment with CP-870,893 in combination with gemcitabine. (A) Median overall survival and progression free survival for patients (n=21) receiving at least one dose of CP-870,893. Relationship between (B) percent change in CA19-9 at end of one cycle of therapy compared to baseline (R = −0.579; p = 0.049), (C) overall survival and tumor response determined by RECIST 1.0 at completion of two cycles of therapy (R = −0.652; p = 0.002), and (D) percent change in total MVPmean for all lesions, primary pancreatic lesion, and all hepatic lesions after two weeks of therapy compared to baseline (R = −0.929; p = 0.007). Best overall tumor response measured by RECIST 1.0 is indicated in (B), (C), and (D) using open circles (partial response), grey circles (stable disease), and black circles (progressive disease).