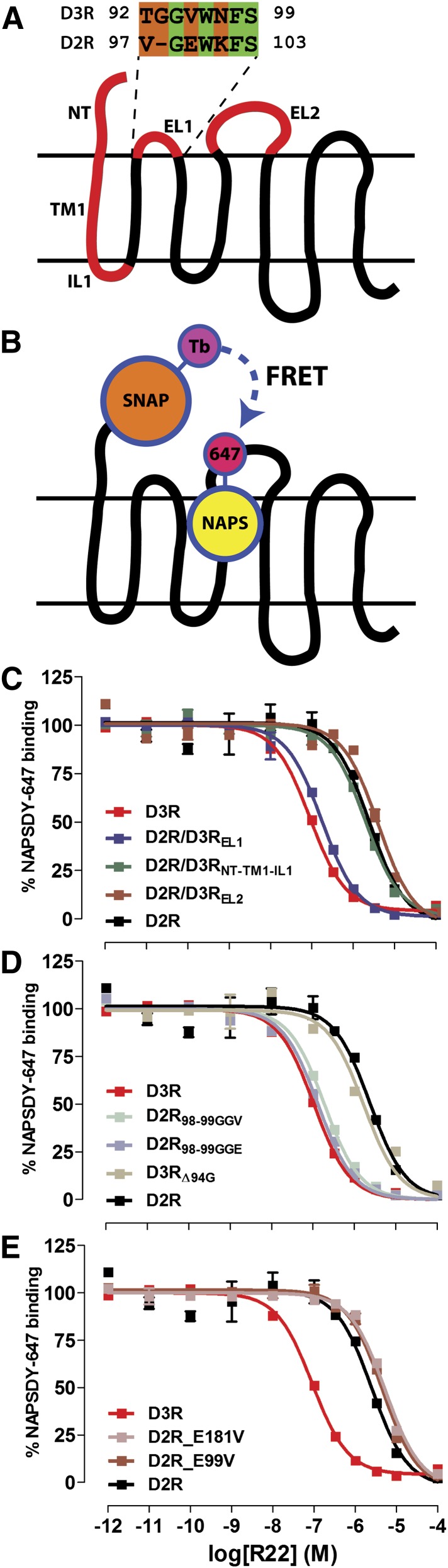
Fig. 6.
The role of EL1 in determining R-22 binding affinity to D3R and D2R. (A) NT-TM1-IL1, EL1, and EL2 of D2R, alone or in combination, were exchanged with that of D3R. (B) Schematic of the HTRF-based binding assay. Receptors were fused with SNAP at their NT, which covalently binds a terbium (Tb)-based donor chromophore that fluorescence resonance energy transfers with an DY-647–conjugated NAPS molecule bound to the OBS. (C) D3R EL1, but not NT-TM1-IL1 or EL2, enhances R-22 binding affinity to D2R similarly to that at wild-type D3R. (D) Addition of a single Gly residue after position 98 of D2R EL1 is sufficient to enhance R-22 affinity similarly to that at D3R. Deletion of Gly94 of D3R reduces R-22 affinity similarly to that at D2R. (E) Mutation of either D2R E99 or E181 to Val has no significant effect on R-22 binding affinity. Binding curves are representative of at least three independent experiments, for which data are summarized in Table 2.