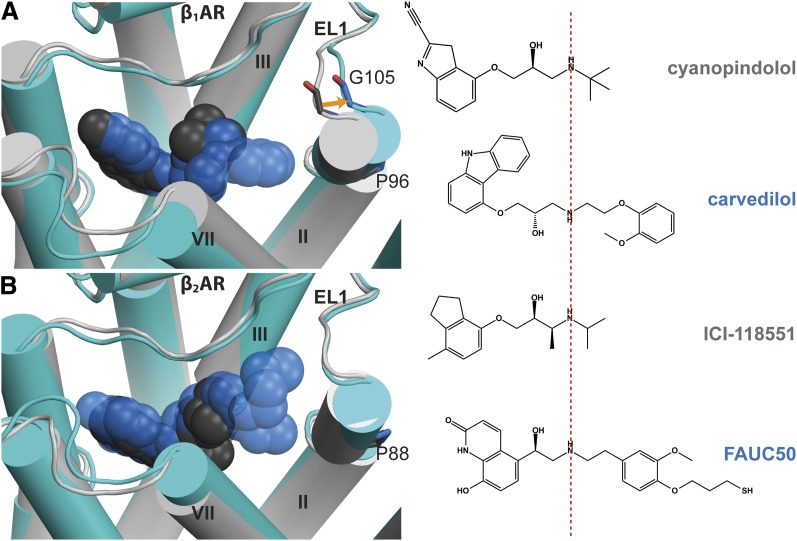
Fig. 7.
Comparison of the structural rearrangement in TM2 and EL1 induced by binding of bulky ligands in β1AR and β2AR. (A) Superposition of the cyanopindolol-bound structure (PDB ID 2VT4, cyanopindolol in dark gray, receptor in light gray) and the carvedilol-bound structure (PDB ID 4AMJ, carvedilol in blue, receptor in cyan) of β1AR shows an outward movement of the extracellular segment of TM2 in the presence of bulky ligand carvedilol, resulting in a 2.3 Å shift of the Cα atom of Gly105 in EL1, and thereby an outward tilting of the extracellular portion of TM2, compared with the cyanopindolol-bound structure. (B) Superposition of the ICI-118551–bound structure (PDB ID 3NY8, ICI-118551 in dark gray, receptor in light gray) and the FAUC50-bound structure (PDB ID 3PDS, FAUC50 in blue, receptor in cyan) of β2AR shows similar conformations of TM2 and EL1 in the presence of these two ligands. The structures were superimposed by the Cα atoms. ICI-118551, (2S,3S)-1-[(7-methyl-2,3-dihydro-1H-inden-4-yl)oxy]-3-(propan-2-ylamino)butan-2-ol.