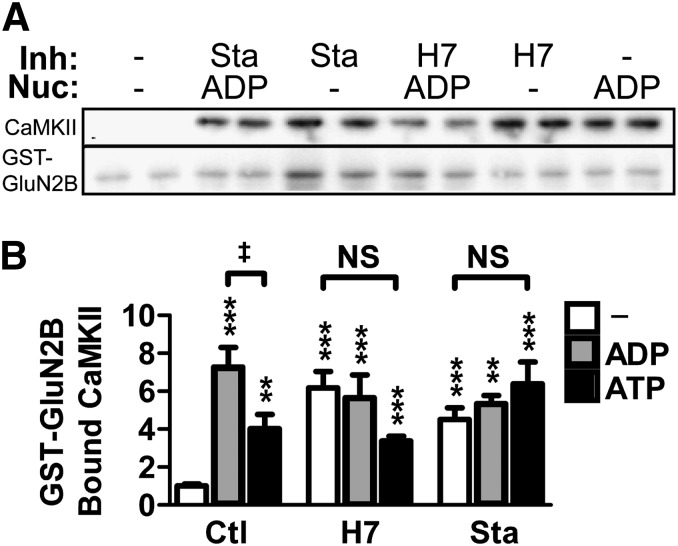
Fig. 3.
H7 and Sta mimic the nucleotide enhancement of CaMKII/GluN2B binding in vitro. CaMKII-binding to GluN2B was assessed by immobilizing GST-tagged GluN2B C-tail to anti–GST-coated plates and adding CaMKII in the presence of Ca2+ and calmodulin. Bound protein was measured by Western blot. (A) Representative Western blot of bound fractions from an in vitro binding assay run in biologic replicates. The membranes were probed for CaMKII (above) and GST-GluN2B (below). Binding reactions contained varied combinations of inhibitors (Inh)—700 μM H7 or 2 μM Sta—and nucleotide (Nuc)—100 μM ADP or ATP. These conditions are indicated above the lanes. (B) Quantification of the Western blot results normalized to no-nucleotide control showing enhancement of binding under all conditions relative to no-nucleotide control (one-way ANOVA; **P < 0.01; ***P < 0.001). No differences existed within H7 or Sta treatments, though binding in the presence of ADP was greater than ATP (one-way ANOVA; ‡P < 0.05). (n = 6–17 per group)