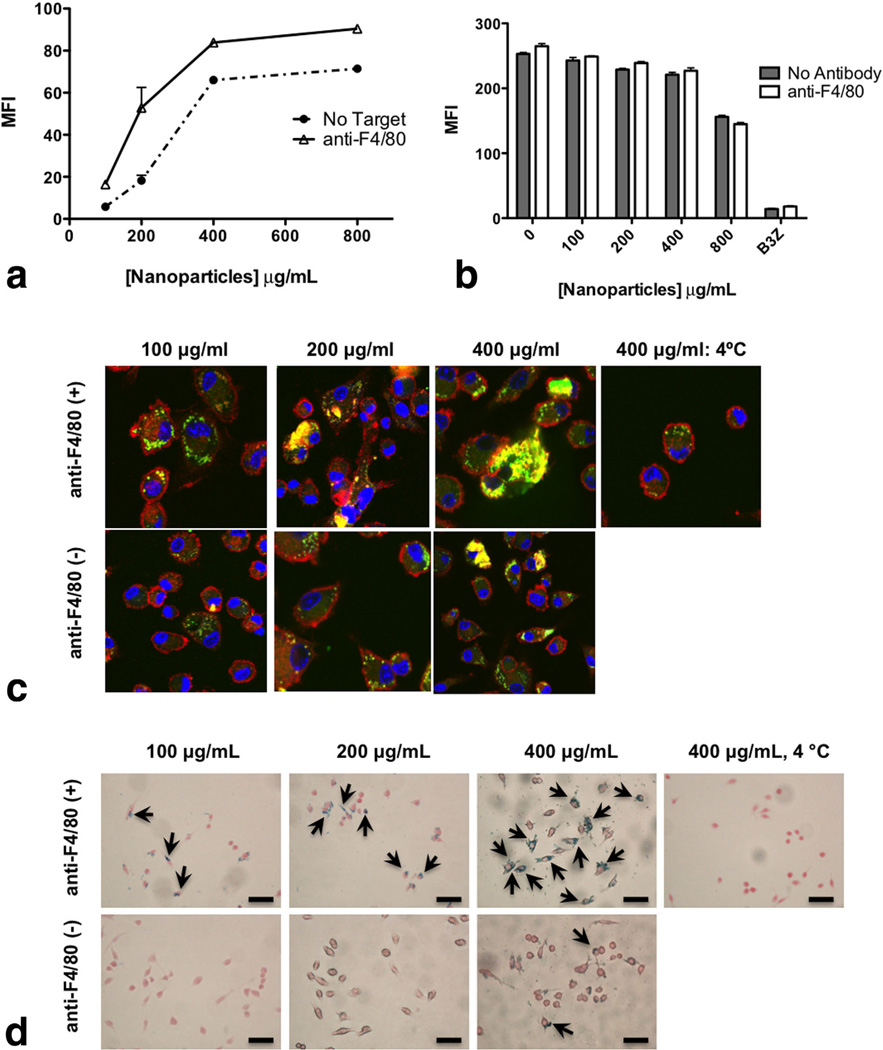
FIG. 4.
a: Nanoparticle internalization (both targeted and nontargeted) by macrophages using flow cytometry. BMDMs were exposed for 2 h to C-6-loaded nanoparticles (100–1000 µg/mL) with or without targeting with 5 µg/mL anti-F4/80 mAb per milligram nanoparticles. After 2 h, the cells were stained with CD11b-APC and fixed. Cells were gated on CD11b+ population and C-6 fluorescence intensity was measured for the gated CD11b+ population. b: Macrophages were assessed for metabolic activity by measuring the uptake of DiI-Ac-LDL in the cells: BMDMs were exposed to different concentrations of nanoparticles (100–800 µg/mL) for 2 h. After 2 h, cells were washed and Dil-Ac-LDL was added at a concentration of 10 µg/mL in each well for 4 h at 37° C. After 4 h, cells were washed and analyzed for fluorescence at λEx=520 nm and λEm=564 nm using flow cytometry. c: Internalization of targeted and nontargeted nanoparticles assessed by confocal microscopy. BMDMs were plated onto cover slips and exposed to C-6-loaded nanoparticles for 2 h. Cells were treated with or without targeting with anti-F4/80 mAb (5 µg/mg nanoparticles). After the indicated times, cells were fixed, permeabilized, and stained with Alexa Fluor® 548 phalloidin to label F-actin (red) and To-Pro-3 to label the nucleus (blue). Cells were visualized under a Zeiss confocal microscope using wavelengths 488, 568, and 633 nm. d: Internalization of targeted and nontargeted SPIO/C6-PLGA nanoparticles assessed by Prussian blue staining. Macrophages were plated onto cover slips and exposed to nanoparticles for 2 h. Cells were treated with or without targeting with anti-F4/80 mAb (5 µg/mg nanoparticles). Cells were then fixed with neutral buffered formalin, washed with PBS, then stained with ferrocyanide/hydrochloric acid mixture, and counterstained with Nuclear Fast Red. Images were obtained with a Nikon (TE2000-U) electron microscope. Scale bars are 100 µm.